Q: Indicate which compound will have highest and lowest Bronsted acidity. 1. Choose the correct option…
A: Acidity is defined as the ability of a molecule to release protons.If a compound easily donates…
Q: Energy is released when hydrogen and oxygen react to produce water. This energy comes from the fact…
A: Given question is related to the calculation of amount of energy released when one mole of water is…
Q: Give the IUPAC name for the following compound. Be sure to use cis/trans, E/Z or R/S where…
A: RULES for IUPAC nomenclature; In first step, we identify functional groups present. We make longest…
Q: A voltaic cell can be prepared from aluminum and copper according to the following reaction…
A: The objective of the question is to determine the actual cell potential.
Q: C. OH H₂SO4 Classification of Reactant: 1° ROH 2º ROH 3° ROH H₂SO4 (circle one): Nucleophilic acid…
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: Draw structures for triammineaquadichlorocobalt(III)chloride, all isomers, and potassium…
A: Given compound formula can represented astriammineaquadichlorocobalt(III)chloride =…
Q: stereochemistry 35 Spp i A. BO C. D. E. OH CI 1. CH3NH₂ 2. LIAH 3. H₂O* H₂O* 1. Mg/THF 2. CO2 3.…
A: Given are organic reactions.Let's see missing products for each reaction in next step.
Q: Draw the major product of the aldol condensation reaction between two equivalents of this ketone…
A: Aldol condensation is base catalyzed condensation between two aldehydes or two ketones to form the…
Q: Given the following electrochemical cell, calculate the potential for the cell in which the…
A: First we need to write the balanced half cell reaction.
Q: The following skeletal oxidation-reduction reaction occurs under basic conditions. Write the…
A: Given,The reaction:
Q: Predict the product(s); CH₂CH3 CH₂CH3 Br₂, hv ?
A: Alkane is a saturated hydrocarbon that has only a single bond.They are less reactive than alkene or…
Q: Three samples of gas each exert 740. mmHg in separate 2L containers. What pressure do they exert if…
A: When gases are combined, the total pressure is the sum of the partial pressure of each gas. This is…
Q: Examine the compounds and then select all the following statements that are correct. DUS 1 2 3 4 A.…
A: The question is based on the concept of organic reactions.We need to distinguish given pair of…
Q: Take a look at this organic reaction: O NAD NADH V CH₂- CH₂-6-0 + CO₂ The reactant molecule is…
A: The given question is
Q: Draw the organic product of the Bronsted acid-base reaction. Include all lone pairs and charges as…
A: Bronsted–Lowry acid base theory : An acid is a proton (H⁺) donor, and a base is a proton acceptor.…
Q: 2. A student dissolves 1.50 moles of magnesium chloride in a certain amount of pure water to make a…
A: Molarity: It is defined as the no. of moles of solute in one litre of solutionMolality: It is…
Q: The logarithm of the molar absorptivity of acetone in ethanol is 2.75 to 366 nm. Calculate the range…
A: We are given that the logarithm of the molar absorptivity of acetone in ethanol is 2.75 to 366 nm.…
Q: For the following multistep synthesis of the target structure on right from the starting structure…
A: The question is based on the concept of organic synthesis.We need to synthesize the product using…
Q: Use the References to access important values if needed for this question. The Solubility Product…
A: The solubility product constant of the calcium sulfate = We have to calculate the molar solubility…
Q: Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side…
A: The reactions in which the carboxylic aicd reacts with an alcohol to form an ester by the loss of…
Q: The reaction below gives two constitutional isomers as products. Draw the structure of the radical…
A: In NBS, 1% bromine is present which undergoes homolytic dissociation to generate bromine radical.…
Q: The complex ion Cu(NH3)2* is formed from a solution that initially contains 1.50 x 10-3 M CUNO3 and…
A:
Q: Propose mechanisms for the formation of both products in the following reaction. CI fer -Br Br HCI…
A: A question based on reaction mechanisms. For the given addition reaction, the respective reaction…
Q: Draw the two possible products of this oxidative cleavage of an alkyne. Ignore any inorganic…
A: Given reaction is an example of oxidative cleavage of alkyne by ozonolysis reaction.In this reaction…
Q: . be p IV Choose the structure that best fits this DEPT-135 spectrum. OH Harma, F 100 60 40 20 A) |…
A:
Q: e the IUPAC name for this compound.
A: The given compound is a hydrocarbon and contains alkene functional groups. The number of the carbon…
Q: H₂N "NH₂
A: Given is organic reaction. The given reaction is,
Q: The [OH(aq)] in 0.10 mol/L NaHCOO (aq) is A. 1.3 x 10-2 mol/L B. 4.2 x 10-3 mol/L C. 2.4 x 10-6…
A: The question is based on the concept of the pH of the solution.It is defined as a negative logarithm…
Q: Aqueous sulfuric acid (H₂SO4) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium…
A: The mass of sulfuric acid = 37.3 gThe mass of sodium hydroxide = 49.2 gThe experimental yield of…
Q: A sample of N₂(g) effuses from a container in 30 s. How long would it take the same amount of NH3(g)…
A:
Q: Name the folloowing complex compounds: [Cu(NH3)4]+2 [PtCl4]-2 Fe(S2CNMe2)3 [Mn(CN)6]-4 [ReH9]-2
A: The nomenclature of coordination compounds follows certain rules established by the IUPAC…
Q: Rank the indicated hydrogen in the following compounds in order from most acidic to least acidic:…
A: The acidity of a compound is determined by the ease with which it can donate a proton (H+). This…
Q: Hi, can someone please help me solve this question? Thank you!!
A: The phase diagram of carbon is a graphical representation of the conditions of temperature and…
Q: What is AH xn for the following reaction? NO2(g) + CO(g) → CO2(g) + NO(g) AH(kJ/mol) Substance NO(g)…
A:
Q: Can the molecule on the right-hand side of this organic reaction be made in good yield from no more…
A: The given structure of the compound is:To identify the reactant/reactants we can do retrosynthesis.
Q: 12. Calculate the energy released, in kJ, when 1.00 mol U-238 isotopes (nuclear mass = 238.05078…
A: the energy released is calculated for alpha decay is given by following equation ∆E = [mr - (mp…
Q: (b) 1.9-BBN 2. H₂O2, KOH
A: Given,The reaction:
Q: What is the abbreviated name of the compound shown below. NH₂ O AMP O ADP O ATP N N py OHOH None are…
A:
Q: O: radical initiator
A: Given datain free radical polymerization, the reactive intermediate is free radical. Here, vinyl…
Q: 1. Give all possible ß-elimination products for the following reactions and circle the major product…
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: Starting from benzene synthesize ONE of the following compounds in good yield using any inorganic…
A: The most common type of reaction in benzene is the electrophilic substitution reaction. The…
Q: :0: :O: H Select to Add Arrows :O: N. 4 H H :CHO H Ï :CI: 1 I I I I I I I I I
A: An arrow always depicts from a region of high electron density to low electron density ; that is…
Q: Aqueous sulfuric acid (H₂SO4) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium…
A: We have given Aqueous sulfuric acid reacts with solid sodium hydroxide to produce aqueous sodium…
Q: When the following skeletal equation is balanced under basic conditions, what are the coefficients…
A: Answer:- This question is answered by using the simple concept of balancing the redox reaction.
Q: Compound A has molecular formula C6H12. Upon treatment with NBS and irradiation with UV light,…
A: N- bromo succinamide (NBS) is a reagent used for allylic bromination.
Q: LOTI SnBu3 Modify the given copy of the electrophile starting material to draw the structure of the…
A: Given is organic reaction.This is coupling reaction.The name of given reaction is Stille coupling…
Q: Where would you expect to find a cysteine unit in the tertiary structure of a water-soluble globular…
A: The objective of the question is to understand the positioning of cysteine in the tertiary structure…
Q: The oxidation of HNO₂ to NO3 in acid solution is a 4-electron transfer reaction described by the…
A: Answer:Here:n is equal to number of electrons transferred from anode to cathode during cell reaction
Q: OCH3 OCH 3
A: NMR spectroscopy is the use of NMR phenomena to study the physical, chemical, and biological…
Q: A compound shows strong, very broad IR absorption in the region 3300-3600 cm and strong, sharp…
A: IR spectroscopy is used to determine the functional groups present in the sample. Different…
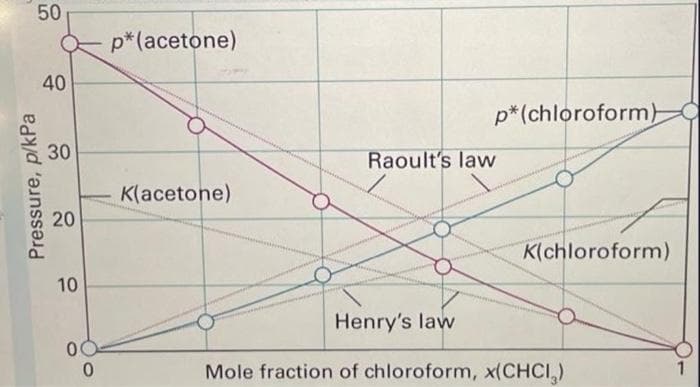
Using the Raoult's law standard state, estimate the activity coefficient of CHCl3 in a solution with χ(CHCl3) = 0.8.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 9 images

- 1-Pentanol to 1-bromopentane Chemicals: - 60ml Conc. Sulfuric Acid - 100ml Saturated Sodium bicarbonate - 65ml 1-Pentanol - 78g sodium bromide - Distilled water - 58.42g 1-Bromopentane 1-Pentanol Sodium Bromide Sulfuric Acid 1-Bromopentane Formula C5H12O NaBr H2SO4 C5H11Br MW (g/mol) 88.15 102.894 98.078 151.04 Density (g/mL) 0.811 3.21 1.84 1.218 Boiling point (*C) 138 1,396 337 130 NaBr(aq) + H2SO4(aq) -> NaHSO4(aq) + HBr(aq) CH3(CH2)4OH(aq) + H+ Br- (aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4OH2 (aq) + Br-(aq) CH3(CH2)4Br(aq) + H2O(aq) How do I calculate the percent yield and identify the limiting reagent?Calculate the missing data in the following table. Compound ΔHvap (kJ/mol) ΔSvap [J/(mol·K)] Boiling Point (°C) hexanoic acid 71.1 105.7 hexane 28.9 85.5 69 or 68.73 formic acid 60.7 100.8 1-hexanol 44.5 157.5 The text states that the magnitude of ΔSvap tends to be similar for a wide variety of compounds. Based on the values in the table, do you agree?Extintion coeficient for Y is 1280
- Use the molar DHf° under the formulas to calculate DH°rxn for the equations as balanced: 1. 2B2H6(g) + 3CO2(g) --> 2B2O3(s) + 3CH4(g) ΔH°rxn = _______ kJ ΔH°f = +36 –394 –1274 –75 kJ/mol exo ? endo_thermic 2. 2P2O5 + 2CaC2 -->P4 + 2CaCO3 + 2CO2 ΔH°rxn = _______ kJ ΔH°f = –1505 –59 ___ –1207 –394 kJ/mol exo ? endo_thermic 3. 2Na2CrO4(s) + 10HCl(g) --> 4NaCl(s) + 3Cl2(g) + Cr2O3(s) + 5H2O(l) ΔH°f = –1342 –92 –411 ___ –1140 –286 kJ/mol ΔH°rxn = _______ kJ exo ? endo_thermicThe normal freezing point of n-octane 1C8H182 is -57 °C. In what temperature range is it a nonspontaneous process?A solution of chloroform (CHCl3) and acetone((CH3)2CO) exhibits a negative deviation from Raoult's law.This result implies that:W. chloroform-chloroform and acetone-acetone interactions are stronger than chloroform-acetone interactions.X. chloroform-chloroformand acetone-acetone interactions are weaker than chloroform-acetone interactions.Y. acetone-acetone interactions are stronger than chloroform-chloroform interactions.Z. acetone-acetone interactions are weaker than chloroform-chloroform interactions. only X is true W and Y are true only W is true X and Z are true only Y is true
- In a beaker, I mix 100 ml of 4 M HCl with 100 ml of distilled water. Both fluids are at 25 C initially. (Cp mix = 0.985 cal/g-C) a. What is the Q required if we want to keep the temperature at 25 C? b. What is the T if its adiabatic? c. What is the molarity of the final mixture?A 1-kg iron bar (c=0.11 kcal/kgC) at 100C is placed in 3.0 kg water at 15C. The temperature of the water increases by A. 0.7C B. 3C C. 5C D. 18CGive the solution with proper sig figs. 4.523 g O2 x ( 1 mol O2 / 32.00 g O2) x (– 533.7 kJ/ 3 mol O2) = – 25.14505313 kJ a. – 25.145 kJ b. – 25.14505313 kJ c. – 25 kJ d. – 25.1 kJ e. none of these f. – 25.15 kJ g. – 2 x 10^1 kJ h. – 3 x 10^1 kJ i. – 25.14 kJ
- Consider the following reaction: NH4Cl--->NH3(g)+HCl(g). The vapor pressure after dissasociation of a certain amount of NH4Cl is 608 kPa at 427 Celsius, but rises to 1115 kPa at 459 Celsius. Calclate Keq, deltarGstandard, and deltarHstandard, all at 427 Celsius. Assume that the vapor is an idela gas and that the enthalpy is indepednet of temperature in the range given.During an experiment to determine the value of R a student collected the following data:Molarity of HCl 3.00MVolume of HCl 20.3mlMass of Mg 0.0411gVolume before equalization chamber 43.1mlVolume after equalization chamber 42.7mlBarometric pressure 759.1mmHgTemperature 21.9Calculate the following information and show all work.Moles of Mg reacted ____Moles of H2 formed ____Vapor pressure of water 19.8mmHgPressure of H2 from dalton’s law ____Volume of H2 ____Temperature(k) ____Calculated R value _______Boiling Point Elevation/Freezing Point Depression T = m K where, for freezing point depression: T = T(pure solvent) - T(solution) and for boiling point elevation: T = T(solution) - T(pure solvent) m = (# moles solute / Kg solvent) Kb = boiling point elevation constant. Kf = freezing point depression constant. Kb and Kf depend only on the SOLVENT. Below are some common values. Use these values for the calculations that follow. Solvent Formula Kb(°C / m) Kf(°C / m) Water H2O 0.512 1.86 Ethanol CH3CH2OH 1.22 1.99 Chloroform CHCl3 3.67 Benzene C6H6 2.53 5.12 Diethyl ether CH3CH2OCH2CH3 2.02

