Using the results of the Arrhenius analysis (Ea = 93.1kJ/mol and A = 4.36 × 101" M · s'), predict the rate constant at 299 K . %3D Express the rate constant in liters per mole-second to three significant figures.
Using the results of the Arrhenius analysis (Ea = 93.1kJ/mol and A = 4.36 × 101" M · s'), predict the rate constant at 299 K . %3D Express the rate constant in liters per mole-second to three significant figures.
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.85E: Which sentence best describes the following reaction? 2H2(g)+O2(g)2H2O(l)+heat a. It is an...
Related questions
Question

Transcribed Image Text:Consider the following reaction:
O3 (g) → O2 (g) +O(g)
You may want to reference (Page) Section 14.5
while completing this problem.
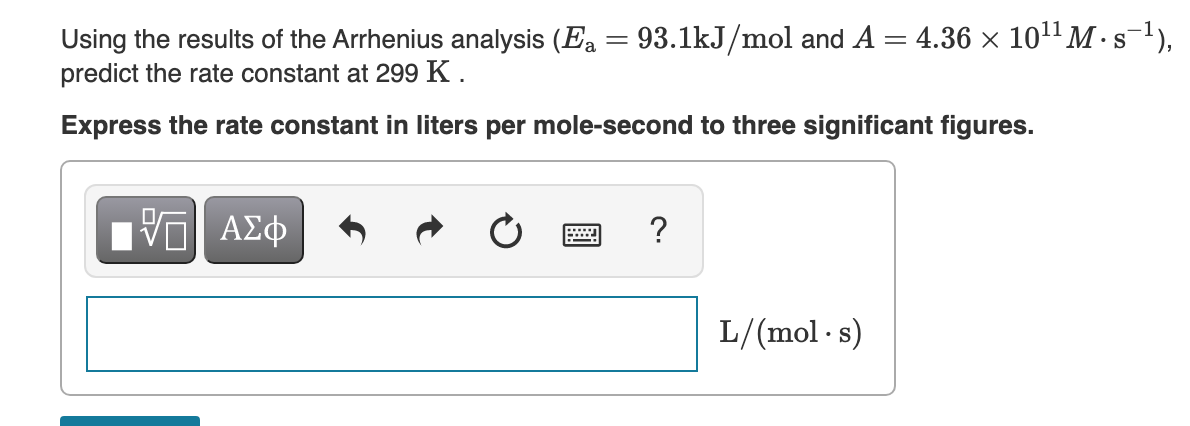
Transcribed Image Text:Using the results of the Arrhenius analysis (Ea = 93.1kJ/mol and A = 4.36 × 101" M · s),
predict the rate constant at 299 K .
S
Express the rate constant in liters per mole-second to three significant figures.
Hν ΑΣφ
L/(mol · s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning