Using the table of the weak base below, you have chosen Ethylamine as your weak base in the buffer solution. You have already added enough of the conjugate acid salt to make the buffer solution concentration at 0.52 M in this salt. The desired pH of the buffer should be equal to 10.1. Values of K, for Some Common Weak Bases Conjugate Acid Name Formula NH CH,NH2 CH&NH; CH;NH, CHN Ammonia Methylamine Ethylamine Aniline Pyridine NH, CH NH, CH,NH; CH,NH, CH,NH 1.8 x 10-5 4.38 x 10-4 5.6 x 10-4 3.8 x 10-10 1.7 x 10-9 2. Compute for pKb. (round off final answer to 2 decimal places)
Using the table of the weak base below, you have chosen Ethylamine as your weak base in the buffer solution. You have already added enough of the conjugate acid salt to make the buffer solution concentration at 0.52 M in this salt. The desired pH of the buffer should be equal to 10.1. Values of K, for Some Common Weak Bases Conjugate Acid Name Formula NH CH,NH2 CH&NH; CH;NH, CHN Ammonia Methylamine Ethylamine Aniline Pyridine NH, CH NH, CH,NH; CH,NH, CH,NH 1.8 x 10-5 4.38 x 10-4 5.6 x 10-4 3.8 x 10-10 1.7 x 10-9 2. Compute for pKb. (round off final answer to 2 decimal places)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 55P
Related questions
Question
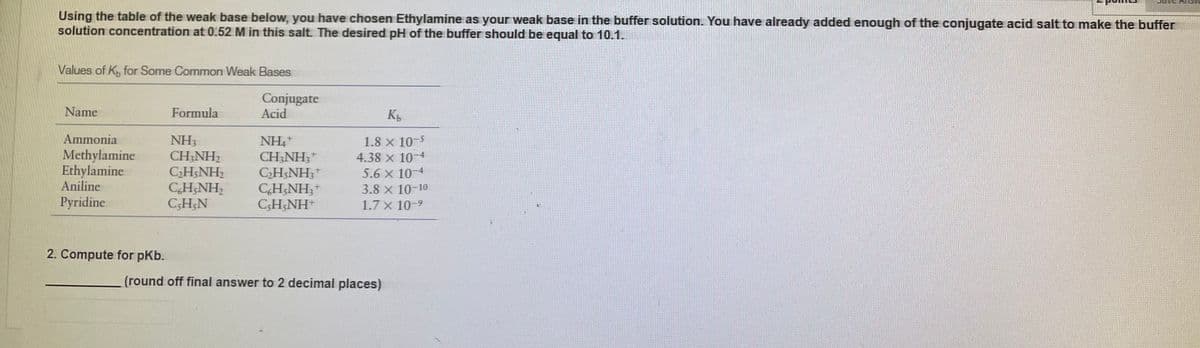
Transcribed Image Text:Using the table of the weak base below, you have chosen Ethylamine as your weak base in the buffer solution. You have already added enough of the conjugate acid salt to make the buffer
solution concentration at 0.52 M in this salt. The desired pH of the buffer should be equal to 10.1.
Values of K, for Some Common Weak Bases
Conjugate
Acid
Name
Formula
K
Ammonia
NH3
CH;NH2
C2H;NH2
CH;NH,
CSH;N
NH,+
CH;NH;*
CH NH;+
CH;NH;+
C;H;NH
1.8 x 10-5
Methylamine
Ethylamine
Aniline
4.38 x 10-4
5.6 x 10-4
3.8 x 10-10
1.7 x 10-9
Pyridine
2. Compute for pKb.
(round off final answer to 2 decimal places)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
