Using this table of ionization constants, calculate the overall equilibrium constant, Koverall, for the reaction. CH3COOH(aq) + S2 (aq) CH3COO¯(aq) + HS¯(aq) Koverall = 5.6 ×105 Incorrect
Using this table of ionization constants, calculate the overall equilibrium constant, Koverall, for the reaction. CH3COOH(aq) + S2 (aq) CH3COO¯(aq) + HS¯(aq) Koverall = 5.6 ×105 Incorrect
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 2.1ACP: Phosphate ions are abundant in cells, both as the ions themselves and as important substituents on...
Related questions
Question
Only typed solution.
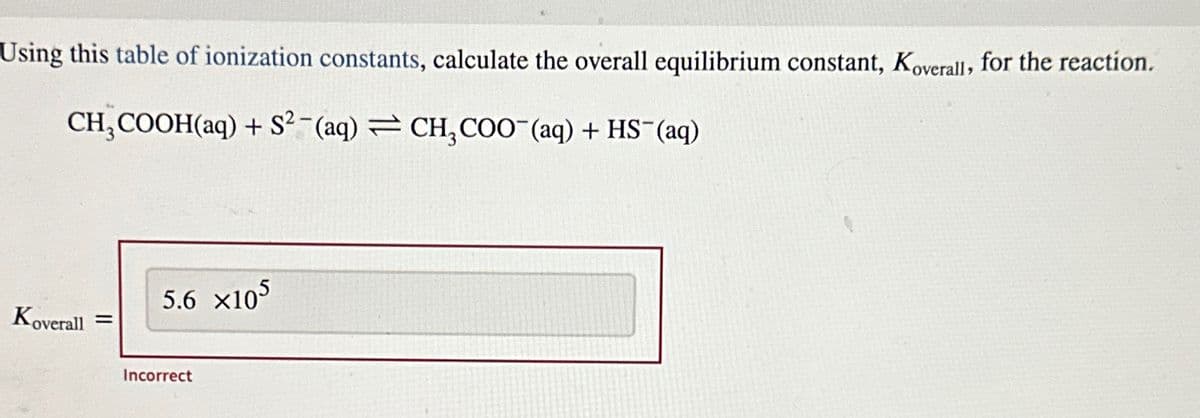
Transcribed Image Text:Using this table of ionization constants, calculate the overall equilibrium constant, Koverall, for the reaction.
CH3COOH(aq) + S2 (aq) CH3COO¯(aq) + HS¯(aq)
Koverall =
5.6 ×105
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning