V Juce asked to determine the percentages of citric acid in the freshly squeezed and bottled lemon following data were obtained. Freshly Squeezed lemon juice Trials Volume of Titrant (ml) 1 25.5 Types of Lemon Juice Freshly Squezzed Bottled 2 25.5 13 25.5 Bottled Lemon juice 12 18.3 1 18.5 Mass of 25 ml (g) 25.6 26.7 3 18.3 Given that the molarity of NaOH is 1.10 M (after standardization with KHP), determine the mass of citric acid in the Bottled juice (8)
V Juce asked to determine the percentages of citric acid in the freshly squeezed and bottled lemon following data were obtained. Freshly Squeezed lemon juice Trials Volume of Titrant (ml) 1 25.5 Types of Lemon Juice Freshly Squezzed Bottled 2 25.5 13 25.5 Bottled Lemon juice 12 18.3 1 18.5 Mass of 25 ml (g) 25.6 26.7 3 18.3 Given that the molarity of NaOH is 1.10 M (after standardization with KHP), determine the mass of citric acid in the Bottled juice (8)
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter30: Nutrition
Section: Chapter Questions
Problem 30.3P: If sodium benzoate, a food preservative, is excreted as such and if calcium propionate, another food...
Related questions
Question
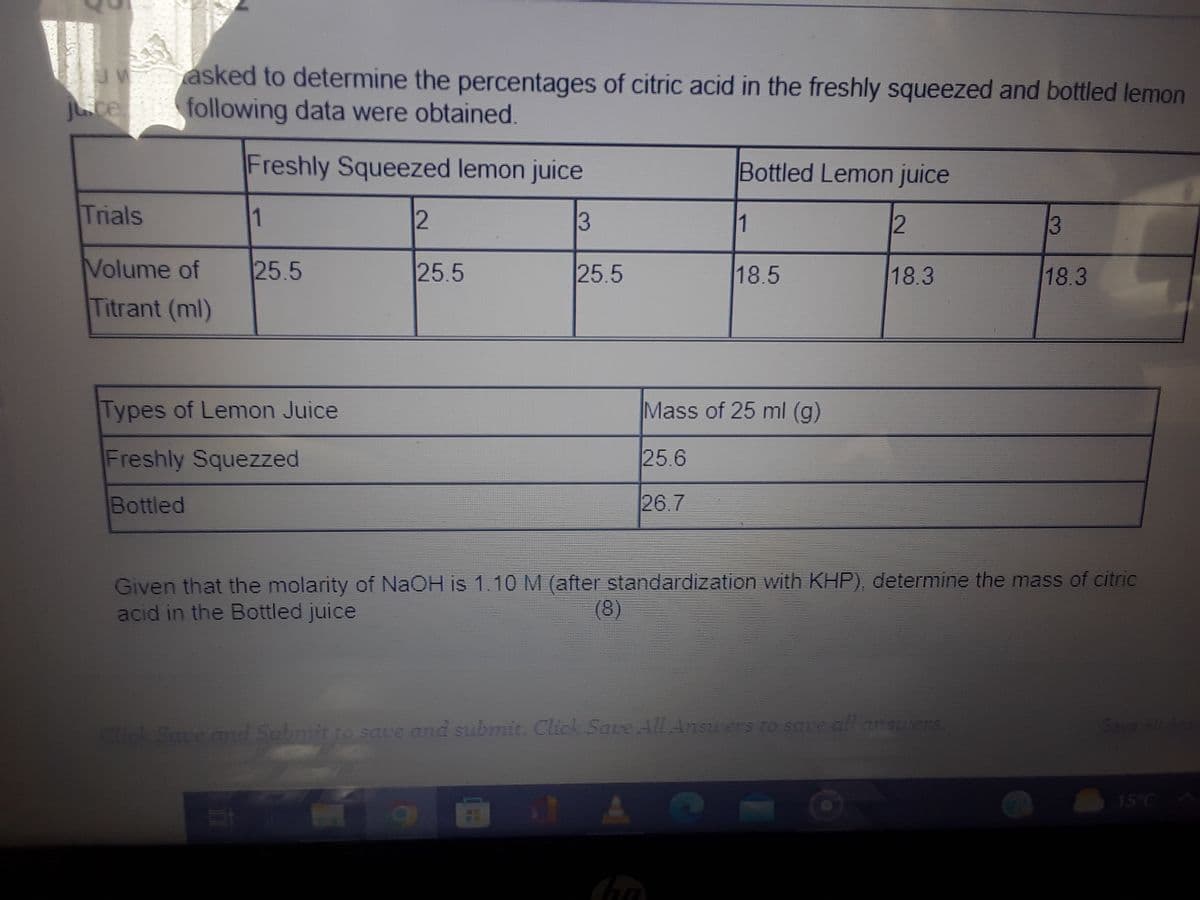
Transcribed Image Text:w
juce
Trials
asked to determine the percentages of citric acid in the freshly squeezed and bottled lemon
following data were obtained.
Freshly Squeezed lemon juice
12
3
Volume of
Titrant (ml)
1
25.5
Types of Lemon Juice
Freshly Squezzed
Bottled
II
25.5
25.5
Bottled Lemon juice
12
1
18.5
Mass of 25 ml (g)
25.6
26.7
18.3
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
3
Given that the molarity of NaOH is 1.10 M (after standardization with KHP), determine the mass of citric
acid in the Bottled juice
(8)
18.3
15°C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning