Van't Hoff studied the temperature dependence of the equilibrium constant, K, using two expressions for the standard Free Energy of a reaction; AG° = - RT In K and AG° = AH° - TAS°. These can be combined in the form of a linear equation, which allows the calculation of the standard enthalpy and entropy changes of a reaction, based on the change of the equilibrium constant, K, with temperature. AH° AS In K = - (*)-(A) R R A reaction is carried out at two temperatures, in an ice bath (0.00°C) and in a boiling water bath (100.00°C). The equilibrium constant, K, is determined at each temperature. At 0.00°C, its value is 5.50E-4. At 100.00°C, its value is 3.22E-4. From this information, determine the values of the following thermodynamic functions for this reaction. Assume that AH° and AS° are independent of temperature, which is generally true over small temperature ranges. What is the value of AH°, in kJ? Submit Answer Tries 0/99 What is the value of AS°, in J/K? Submit Answer Tries 0/99
Van't Hoff studied the temperature dependence of the equilibrium constant, K, using two expressions for the standard Free Energy of a reaction; AG° = - RT In K and AG° = AH° - TAS°. These can be combined in the form of a linear equation, which allows the calculation of the standard enthalpy and entropy changes of a reaction, based on the change of the equilibrium constant, K, with temperature. AH° AS In K = - (*)-(A) R R A reaction is carried out at two temperatures, in an ice bath (0.00°C) and in a boiling water bath (100.00°C). The equilibrium constant, K, is determined at each temperature. At 0.00°C, its value is 5.50E-4. At 100.00°C, its value is 3.22E-4. From this information, determine the values of the following thermodynamic functions for this reaction. Assume that AH° and AS° are independent of temperature, which is generally true over small temperature ranges. What is the value of AH°, in kJ? Submit Answer Tries 0/99 What is the value of AS°, in J/K? Submit Answer Tries 0/99
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 32PS
Related questions
Question
100%
Van’t Hoff equation at two separate temperatures; I have an idea of where to start but still am confused
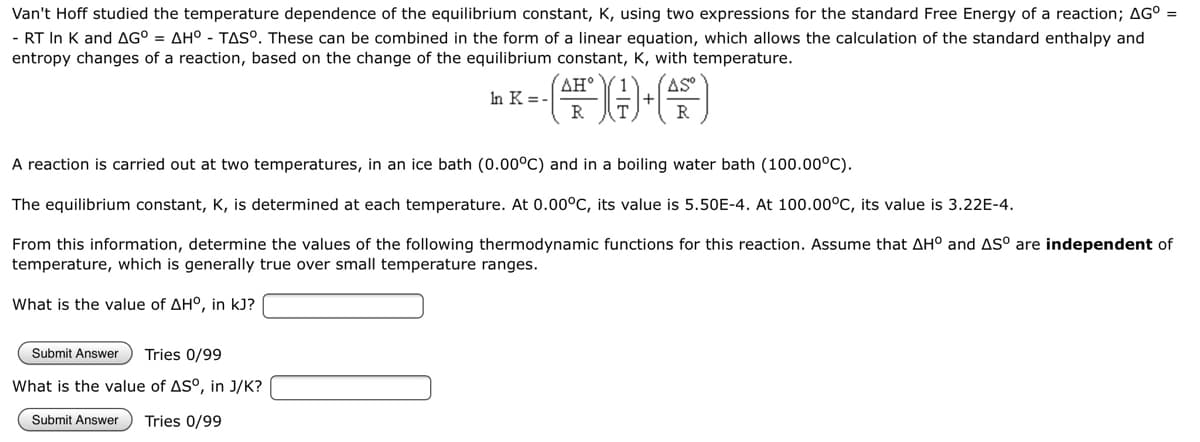
Transcribed Image Text:Van't Hoff studied the temperature dependence of the equilibrium constant, K, using two expressions for the standard Free Energy of a reaction; AG° =
- RT In K and AG° = AH° - TAS°. These can be combined in the form of a linear equation, which allows the calculation of the standard enthalpy and
entropy changes of a reaction, based on the change of the equilibrium constant, K, with temperature.
AH°
AS
In K = -
(FDG)
A reaction is carried out at two temperatures, in an ice bath (0.00°C) and in a boiling water bath (100.00°C).
The equilibrium constant, K, is determined at each temperature. At 0.00°C, its value is 5.50E-4. At 100.00°C, its value is 3.22E-4.
From this information, determine the values of the following thermodynamic functions for this reaction. Assume that AH° and AS° are independent of
temperature, which is generally true over small temperature ranges.
What is the value of AH°, in kJ?
Submit Answer Tries 0/99
What is the value of AS°, in J/K?
Submit Answer Tries 0/99
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning