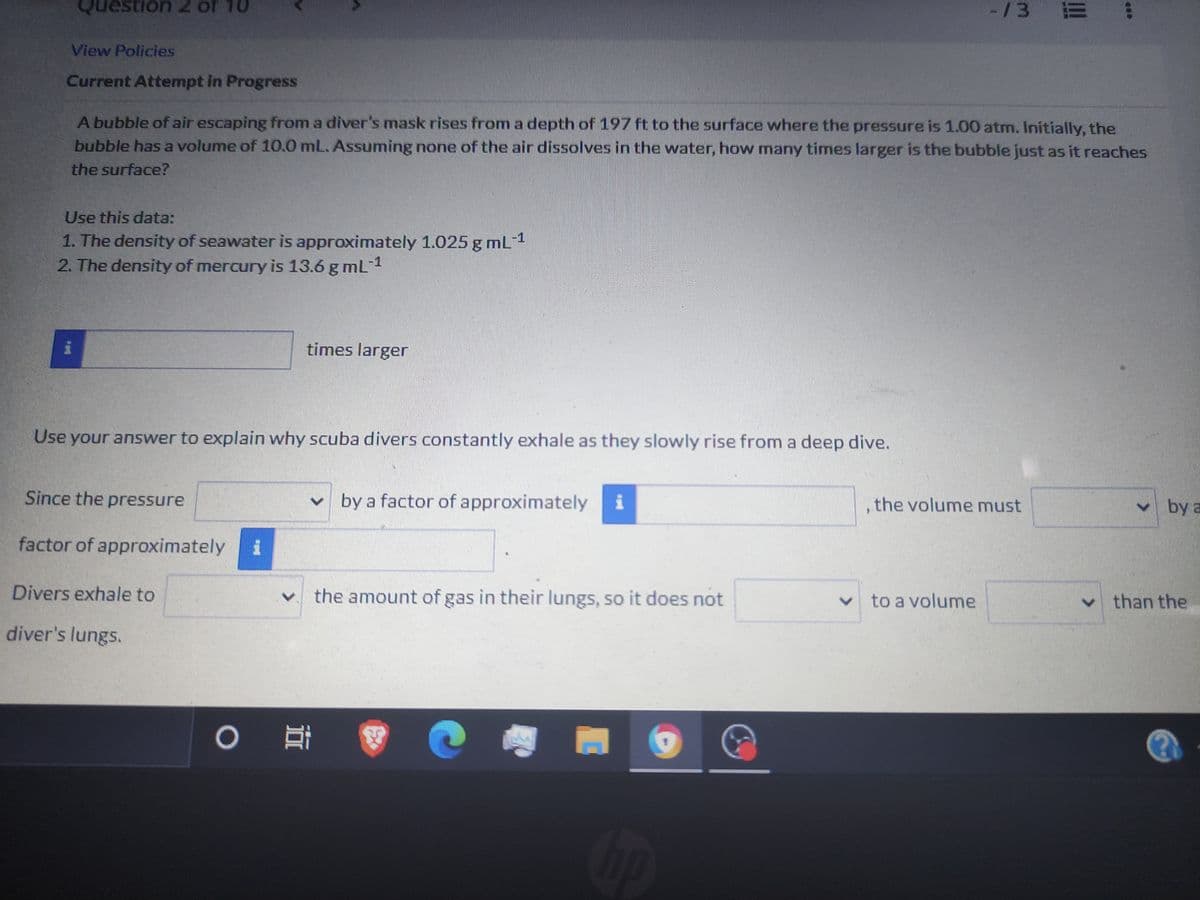
View Policies Current Attempt in Progress A bubble of air escaping from a diver's mask rises from a depth of 197 ft to the surface where the pressure is 1.00 atm. Initially, the bubble has a volume of 10.0 mL. Assuming none of the air dissolves in the water, how many times larger is the bubble just as it reaches the surface? Use this data: 1. The density of seawater is approximately 1.025 g mL 1 2. The density of mercury is 13.6 g mL-1 times larger Use your answer to explain why scuba divers constantly exhale as they slowly rise from a deep dive. Since the pressure by a factor of approximately i ,the volume must ba factor of approximately
View Policies Current Attempt in Progress A bubble of air escaping from a diver's mask rises from a depth of 197 ft to the surface where the pressure is 1.00 atm. Initially, the bubble has a volume of 10.0 mL. Assuming none of the air dissolves in the water, how many times larger is the bubble just as it reaches the surface? Use this data: 1. The density of seawater is approximately 1.025 g mL 1 2. The density of mercury is 13.6 g mL-1 times larger Use your answer to explain why scuba divers constantly exhale as they slowly rise from a deep dive. Since the pressure by a factor of approximately i ,the volume must ba factor of approximately
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.93PAE: 93 The complete combustion of octane can be used as a model for the burning of gasoline:...
Related questions
Question
Transcribed Image Text:Question 2 of 10
-/3
View Policies
Current Attempt in Progress
A bubble of air escaping from a diver's mask rises from a depth of 197 ft to the surface where the pressure is 1.00 atm. Initially, the
bubble has a volume of 10.0 mL. Assuming none of the air dissolves in the vwater, how mnany times larger is the bubble just as it reaches
the surface?
Use this data:
1. The density of seawater is approximately 1.025 g mL1
2. The density of mercury is 13.6 g mL1
times larger
Use your answer to explain why scuba divers constantly exhale as they slowly rise from a deep dive.
Since the pressure
v by a factor of approximately i
, the volume must
v by a
factor of approximately
Divers exhale to
the amount of gas in their lungs, so it does not
v to a volume
vthan the
diver's lungs.
!!
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning