voltage (V) if an unknown metal (M) was electrodeposited from an aqueous solution containing M2X1 (where X is the anion) with a supplied current of 4.1 A for 9 h, consuming
voltage (V) if an unknown metal (M) was electrodeposited from an aqueous solution containing M2X1 (where X is the anion) with a supplied current of 4.1 A for 9 h, consuming
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 103QAP: An alloy made up of tin and copper is prepared by simultaneously electroplating the two metals from...
Related questions
Question
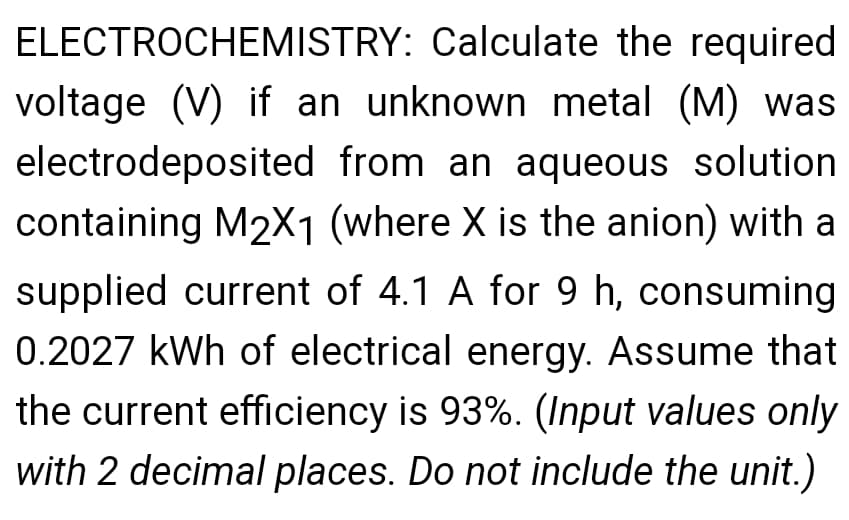
Transcribed Image Text:ELECTROCHEMISTRY: Calculate the required
voltage (V) if an unknown metal (M) was
electrodeposited from an aqueous solution
containing M2X1 (where X is the anion) with a
supplied current of 4.1 A for 9 h, consuming
0.2027 kWh of electrical energy. Assume that
the current efficiency is 93%. (Input values only
with 2 decimal places. Do not include the unit.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning