Volume in ml of 2.00 × 10³ M Fe(NO₂) 5 5 5 5 5 Known 1.239 A Test tube # 1 2 3 4 5 2 3 4 5 Test tube absorbance 0.182 A 0.284 A 0.395 A 0.518 A Beers Lambert Law A=abc CALCULATIONS: 1. Calculate the concentration from the known Fe(SCN)²+ test tube. Use this value to determine the absorbtivity coefficient for the Fe(SCN)²+. Show the complete set of calculations for test tube #1, all initial concentrations, and the ice table. Fill in the rest of the provided data table. a. Prepare a solution of Fe(SCN)² of known concentration by combining 10.0ml of 0.200 M Fe(NO3)3 in 1 M HNO3 measured with your 10ml graduated cylinder, 2.00 ml of 0.0020 M KSCN measured by buret, and 8.00 ml of distilled water measured by buret. In this solution, the concentration of Fe³* is much greater than that of SCN. Concentration of Fe(NO3)3 is 2.00X10^-3 Concentration of KSCN is 2.00X10^-3 Volume in ml of 2.00 x 10³ M KSCN 1 2 3 4 5 1 0.105 A Volume of Water 4 3 2 1 0
Volume in ml of 2.00 × 10³ M Fe(NO₂) 5 5 5 5 5 Known 1.239 A Test tube # 1 2 3 4 5 2 3 4 5 Test tube absorbance 0.182 A 0.284 A 0.395 A 0.518 A Beers Lambert Law A=abc CALCULATIONS: 1. Calculate the concentration from the known Fe(SCN)²+ test tube. Use this value to determine the absorbtivity coefficient for the Fe(SCN)²+. Show the complete set of calculations for test tube #1, all initial concentrations, and the ice table. Fill in the rest of the provided data table. a. Prepare a solution of Fe(SCN)² of known concentration by combining 10.0ml of 0.200 M Fe(NO3)3 in 1 M HNO3 measured with your 10ml graduated cylinder, 2.00 ml of 0.0020 M KSCN measured by buret, and 8.00 ml of distilled water measured by buret. In this solution, the concentration of Fe³* is much greater than that of SCN. Concentration of Fe(NO3)3 is 2.00X10^-3 Concentration of KSCN is 2.00X10^-3 Volume in ml of 2.00 x 10³ M KSCN 1 2 3 4 5 1 0.105 A Volume of Water 4 3 2 1 0
Chapter28: Atomic Spectroscopy
Section: Chapter Questions
Problem 28.4QAP
Related questions
Question
6
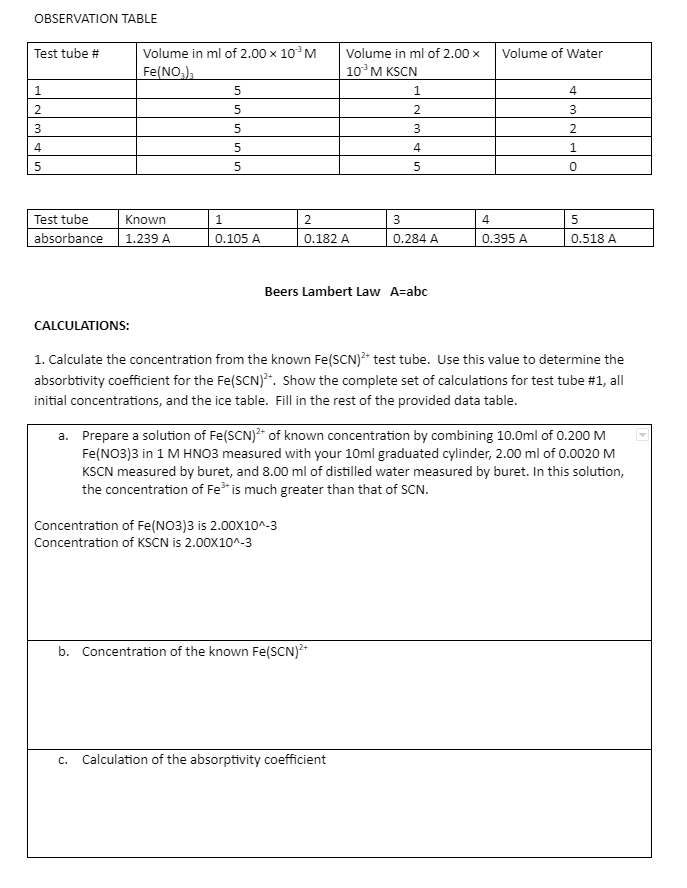
Transcribed Image Text:OBSERVATION TABLE
Test tube #
1
Volume in ml of 2.00 x 10³ M
Fe(NO3)3
5
5
5
5
5
Known
1.239 A
Volume of Water
4
2
3
3
2
4
1
5
0
2
3
4
Test tube
absorbance
5
0.518 A
0.182 A
0.284 A
0.395 A
Beers Lambert Law A=abc
CALCULATIONS:
1. Calculate the concentration from the known Fe(SCN)²+ test tube. Use this value to determine the
absorbtivity coefficient for the Fe(SCN)2+. Show the complete set of calculations for test tube #1, all
initial concentrations, and the ice table. Fill in the rest of the provided data table.
a.
Prepare a solution of Fe(SCN)²+ of known concentration by combining 10.0ml of 0.200 M
Fe(NO3)3 in 1 M HNO3 measured with your 10ml graduated cylinder, 2.00 ml of 0.0020 M
KSCN measured by buret, and 8.00 ml of distilled water measured by buret. In this solution,
the concentration of Fe³+ is much greater than that of SCN.
Concentration of Fe(NO3)3 is 2.00X10^-3
Concentration of KSCN is 2.00X10^-3
b. Concentration of the known Fe(SCN)²+
c. Calculation of the absorptivity coefficient
1
0.105 A
Volume in ml of 2.00 x
10³ M KSCN
1
2
3
4
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

