Volume of trapped gas = 88.57 mL - moles of O2 = 0.00359 - Pressure of trapped gas = 756 mmHg - Partial pressure collected O2 =732.2 mm Hg. - temperature = 298.15 K d. From these results, calculate an experimental value for the ideal gas constant, R in L atm mol–1 K–1. e. Using 0.0821 as the true value for the gas constant, calculate the % error of the experiment to the nearest whole number.
Volume of trapped gas = 88.57 mL - moles of O2 = 0.00359 - Pressure of trapped gas = 756 mmHg - Partial pressure collected O2 =732.2 mm Hg. - temperature = 298.15 K d. From these results, calculate an experimental value for the ideal gas constant, R in L atm mol–1 K–1. e. Using 0.0821 as the true value for the gas constant, calculate the % error of the experiment to the nearest whole number.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter19: The Representative Elements
Section: Chapter Questions
Problem 92CWP
Related questions
Question
-Volume of trapped gas = 88.57 mL
- moles of O2 = 0.00359
- Pressure of trapped gas = 756 mmHg
- Partial pressure collected O2 =732.2 mm Hg.
- temperature = 298.15 K
d. From these results, calculate an experimental value for the ideal gas constant,
R in L atm mol–1 K–1.
e. Using 0.0821 as the true value for the gas constant, calculate the % error of
the experiment to the nearest whole number.
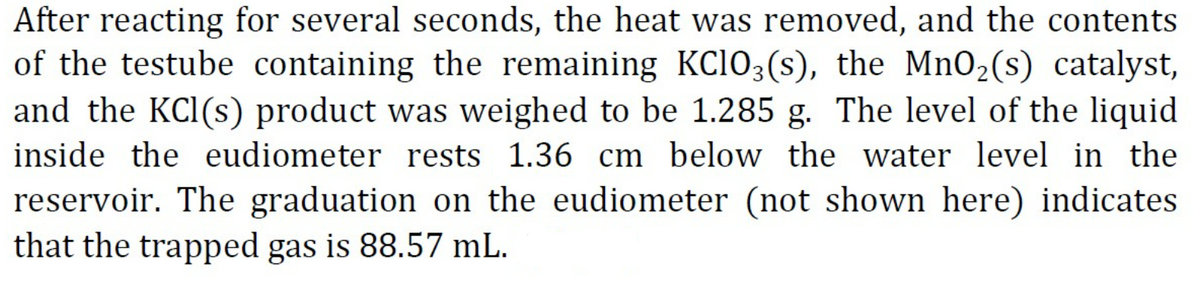
Transcribed Image Text:After reacting for several seconds, the heat was removed, and the contents
of the testube containing the remaining KC103(s), the MnO2(s) catalyst,
and the KCl(s) product was weighed to be 1.285 g. The level of the liquid
inside the eudiometer rests 1.36 cm below the water level in the
reservoir. The graduation on the eudiometer (not shown here) indicates
that the trapped gas is 88.57 mL.
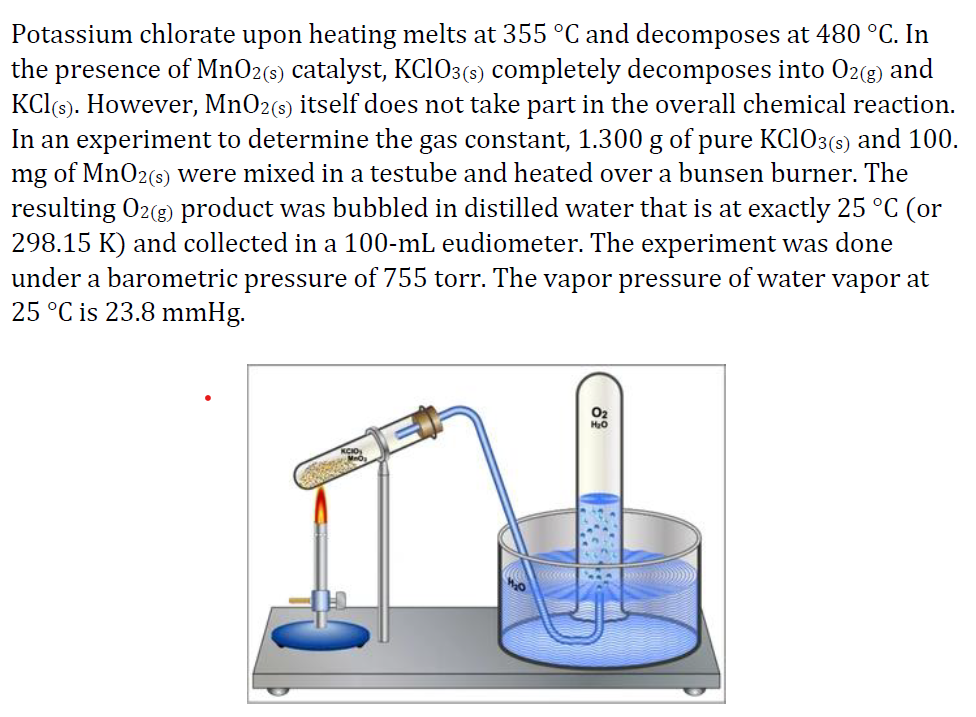
Transcribed Image Text:Potassium chlorate upon heating melts at 355 °C and decomposes at 480 °C. In
the presence of MnO2(s) catalyst, KCIO3(5) completely decomposes into 02(8) and
KCl(s). However, MnO2(s) itself does not take part in the overall chemical reaction.
In an experiment to determine the gas constant, 1.300 g of pure KC1O3(s) and 100.
mg of MnO2(s) were mixed in a testube and heated over a bunsen burner. The
resulting O2(g) product was bubbled in distilled water that is at exactly 25 °C (or
298.15 K) and collected in a 100-mL eudiometer. The experiment was done
under a barometric pressure of 755 torr. The vapor pressure of water vapor at
25 °C is 23.8 mmHg.
O2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning