vorites here, select = then , and drag o the Favorites bar A 70.3 g piece of metal at 88.0°C is placed in 115 g of water at 21.0°C contained in a calorimeter. The metal and water come to the same temperature at 25.1°C. How much heat (in J) did the metal give up to the water? (Assume the specific heat of water is 4.18 J/g.°C across the temperature range.) 4.0 What is the specific heat (in J/g.°C) of the metal? 4.0 J/g °C Supporting Materials Periodic Table Constants and I Supplemental Data Factors Additional Materials Section 5.2 Show My Work (Required) ? What steps or reasoning did you use? Your work counts towards your score. Uploaded File (10 file maximum) You can submit show my work an unlimited number of times. No Files to Display O Upload File 5:36 PM 3/30/2020 W. Type here to search Gp end home delete prt sc to 144 f5 f4 backspace lock
vorites here, select = then , and drag o the Favorites bar A 70.3 g piece of metal at 88.0°C is placed in 115 g of water at 21.0°C contained in a calorimeter. The metal and water come to the same temperature at 25.1°C. How much heat (in J) did the metal give up to the water? (Assume the specific heat of water is 4.18 J/g.°C across the temperature range.) 4.0 What is the specific heat (in J/g.°C) of the metal? 4.0 J/g °C Supporting Materials Periodic Table Constants and I Supplemental Data Factors Additional Materials Section 5.2 Show My Work (Required) ? What steps or reasoning did you use? Your work counts towards your score. Uploaded File (10 file maximum) You can submit show my work an unlimited number of times. No Files to Display O Upload File 5:36 PM 3/30/2020 W. Type here to search Gp end home delete prt sc to 144 f5 f4 backspace lock
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
Section: Chapter Questions
Problem SI6RE
Related questions
Question
Transcribed Image Text:vorites here, select = then , and drag o the Favorites bar
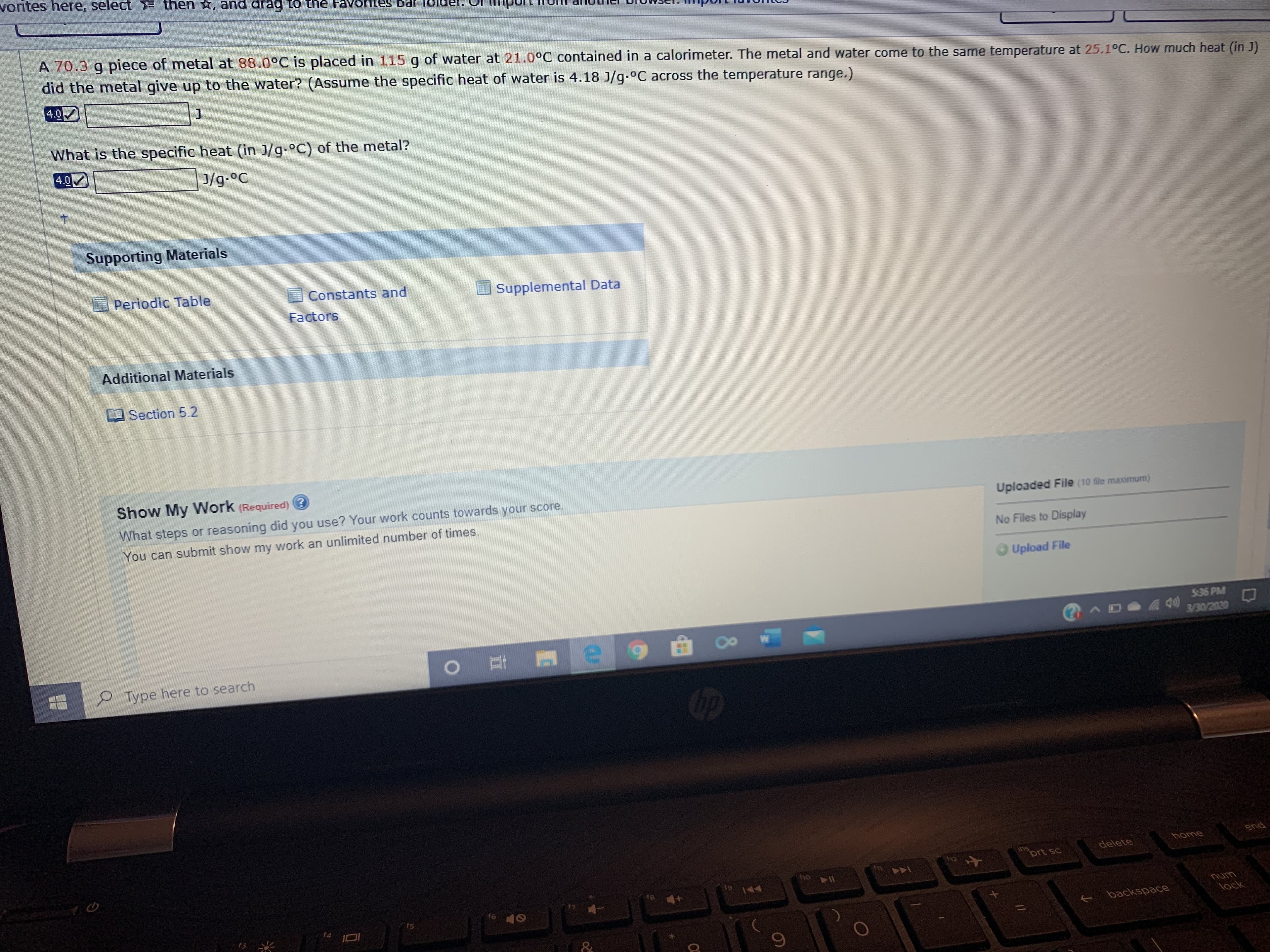
A 70.3 g piece of metal at 88.0°C is placed in 115 g of water at 21.0°C contained in a calorimeter. The metal and water come to the same temperature at 25.1°C. How much heat (in J)
did the metal give up to the water? (Assume the specific heat of water is 4.18 J/g.°C across the temperature range.)
4.0
What is the specific heat (in J/g.°C) of the metal?
4.0
J/g °C
Supporting Materials
Periodic Table
Constants and
I Supplemental Data
Factors
Additional Materials
Section 5.2
Show My Work (Required) ?
What steps or reasoning did you use? Your work counts towards your score.
Uploaded File (10 file maximum)
You can submit show my work an unlimited number of times.
No Files to Display
O Upload File
5:36 PM
3/30/2020
W.
Type here to search
Gp
end
home
delete
prt sc
to 144
f5
f4
backspace
lock
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning