Water at 50°C and 1 atm pressure is heated to 100°C at constant pressure. Using coefficient of volume expansion data, determine the change in the density of water.
Water at 50°C and 1 atm pressure is heated to 100°C at constant pressure. Using coefficient of volume expansion data, determine the change in the density of water.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
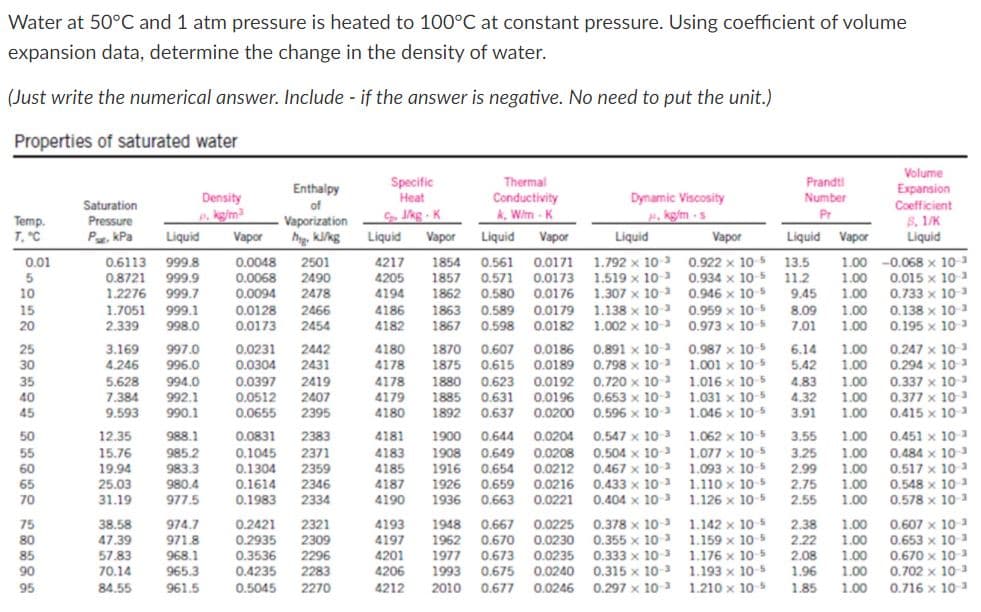
Transcribed Image Text:Water at 50°C and 1 atm pressure is heated to 100°C at constant pressure. Using coefficient of volume
expansion data, determine the change in the density of water.
(Just write the numerical answer. Include - if the answer is negative. No need to put the unit.)
Properties of saturated water
Volume
Specific
Thermal
Prandti
Enthalpy
Expansion
Number
Density
P. kgima
Conductivity
k, Wim - K
Dynamic Viscosity
H. kgfm -s
Heat
Saturation
of
Coefficient
G Jkg - K
Pr
8, 1/K
Liquid
Pressure
Temp.
T. "C
Vaporization
hng, kkg
Pat, kPa
Liquid
Vapor
Liquid
Vapor
Liquid
Vapor
Liquid
Vapor
Liquid Vapor
1854
1857
1862
0.561
0.571
0.580
1.792 x 10-3
1.519 x 10-3
0.0176 1.307 x 10-3
1.138 x 10-3
0.0182 1.002 x 10-3 0.973 x 10-5
1.00 -0.068 x 10-3
0.015 x 10
0.733 x 10-
0.138 x 10 3
0.195 x 10 3
4217
0.0171
0.922 x 10 5
0.934 x 10-5
0.946 x 10
0.959 x 10
0.01
0.6113 999.8
999.9
0.8721
1.2276 999.7
0.0048
2501
2490
13.5
0.0068
4205
0.0173
11.2
1.00
10
0.0094
2478
4194
9.45
1.00
0.0128
0.0173
2466
2454
8.09
7.01
15
1.7051 999.1
4186
1863
0.589
0.0179
1.00
20
2.339
998.0
4182
1867
0.598
1.00
1870
1875
0.0231
0.0304
0.0397
0.0512
0.0655
0.0186 0.891 x 10-3 0.987 x 10-5
1.001 x 105
0.0192 0.720 x 10-3 1.016 x 10-5
1.031 x 10 5
1.046 x 10-5
0.247 x 10 3
0.294 x 10 3
25
3.169
997.0
2442
2431
4180
0.607
6.14
1.00
30
4.246
996.0
4178
0.615
0.0189
0.798 x 10 3
5.42
1.00
35
40
994.0
992.1
2419
2407
2395
0.623
0.631
0.337 x 10 3
0.377 x 10 3
0.415 x 10 3
5.628
4178
1880
4.83
1.00
0.0196 0.653 x 10-3
0.596 x 10-3
7.384
4179
1885
4.32
1.00
45
9.593
990.1
4180
1892
0.637
0.0200
3.91
1.00
0.0831
0.1045
0.1304
0.1614
0.547 x 10-3 1.062 x 10-5
0.504 x 10-
0.467 x 10-3 1.093 x 10 5
0.433 x 10-3 1.110 x 10-5
0.404 x 10 3
0.451 x 10
0.484 x 10-3
0.517 x 10 3
0.548 x 10 3
0.578 x 10 3
50
12.35
988.1
2383
4181
1900
0.644
0.0204
3.55
1.00
55
15.76
985.2
2371
4183
1.077 x 10-5
1908
1916
1926
1936
0.649
0.654
0.659
0.663
0.0208
3.25
1.00
60
65
2359
2346
2334
19.94
983.3
4185
4187
2.99
2.75
1.00
1.00
25.03
980.4
977.5
0.0216
70
31.19
0.1983
4190
0.0221
1.126 x 10-5
2.55
1.00
0.378 x 10 3
0.355 x 10 3
0.333 x 10-3 1.176 x 10-5
0.315 x 10-3 1.193 x 10 5
1.142 x 10-5
1.159 x 10 5
0.607 x 10
0.653 x 10 3
0.670 x 10-3
0.702 x 10-
0.716 x 10-
75
38.58
2321
974.7
971.8
0.2421
0.2935
4193
1948
0.667
0.0225
0.0230
0.0235
2.38
1.00
80
47.39
2309
2296
2283
2270
4197
1962 0.670
1977
0.673
1993
0.675
0.677
2010
2.22
1.00
1.00
1.00
85
57.83
70.14
968.1
0.3536
4201
2.08
90
965.3
0.4235
4206
0.0240
1.96
95
84.55
961.5
0.5045
4212
0.0246
0.297 x 10-3
1.210 x 10-5
1.85
1.00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY