water molecule consists of an oxygen atom with two hydrogen atoms bound to it. The angle between the two bonds is 106◦ If each bond is 0.128 nm long, how far from the oxygen atom is the center of mass of the molecule? Take the mass of an oxygen atom to be 16 times the mass of a hydrogen atom. Answer in units of nm.
water molecule consists of an oxygen atom with two hydrogen atoms bound to it. The angle between the two bonds is 106◦ If each bond is 0.128 nm long, how far from the oxygen atom is the center of mass of the molecule? Take the mass of an oxygen atom to be 16 times the mass of a hydrogen atom. Answer in units of nm.
University Physics Volume 3
17th Edition
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:William Moebs, Jeff Sanny
Chapter9: Condensed Matter Physics
Section: Chapter Questions
Problem 64P: If there is one free electron per atom of copper, what is the electron number density of this metal?
Related questions
Question
A water molecule consists of an oxygen atom
with two hydrogen atoms bound to it. The
angle between the two bonds is 106◦
If each bond is 0.128 nm long, how far from
the oxygen atom is the center of mass of the
molecule? Take the mass of an oxygen atom
to be 16 times the mass of a hydrogen atom.
Answer in units of nm.
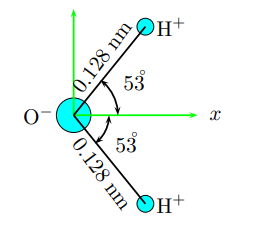
Transcribed Image Text:OH+
53
53
0.128 nm
0.128 nm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning