We will be using the following metals as electrodes for our Batteries: Mg, Al, Cu, Zn Based on their Atomic Size and Electronegativity, which two combinations of metals will give the MOST potentail voltage and which should give the LEAST. IA VIIIA He 53 37 IIA IIA IVA VA VIA VIIA Li 152 Be 11 N F Ne 88 77 75 73 71 69 Na 186 Mg 160 AI 143 SI 117 P 110 CI Ar 97 104 231 197 Ga 153 Ge 122 As 121 Se 117 Br Kr 109 114 K CaO Са 244 Sn 158 215 Sb In 167 Te 137 Xe 130 141 133 Rb Sr 202 TI 171 Pb 175 Po 140 At Rn 140 217 Bi 146 140 Cs Ba Fr Ra We can predict and calculate "potential" voltage by finding the difference in potential between a cathode and anode. To do this simply subtract the "potential" for each metal : Voltage= Cathode - Anode Question: Use the potentials listed in the table to check your o o ol
We will be using the following metals as electrodes for our Batteries: Mg, Al, Cu, Zn Based on their Atomic Size and Electronegativity, which two combinations of metals will give the MOST potentail voltage and which should give the LEAST. IA VIIIA He 53 37 IIA IIA IVA VA VIA VIIA Li 152 Be 11 N F Ne 88 77 75 73 71 69 Na 186 Mg 160 AI 143 SI 117 P 110 CI Ar 97 104 231 197 Ga 153 Ge 122 As 121 Se 117 Br Kr 109 114 K CaO Са 244 Sn 158 215 Sb In 167 Te 137 Xe 130 141 133 Rb Sr 202 TI 171 Pb 175 Po 140 At Rn 140 217 Bi 146 140 Cs Ba Fr Ra We can predict and calculate "potential" voltage by finding the difference in potential between a cathode and anode. To do this simply subtract the "potential" for each metal : Voltage= Cathode - Anode Question: Use the potentials listed in the table to check your o o ol
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter32: Voltaic Cell Measurements
Section: Chapter Questions
Problem 2ASA
Related questions
Question
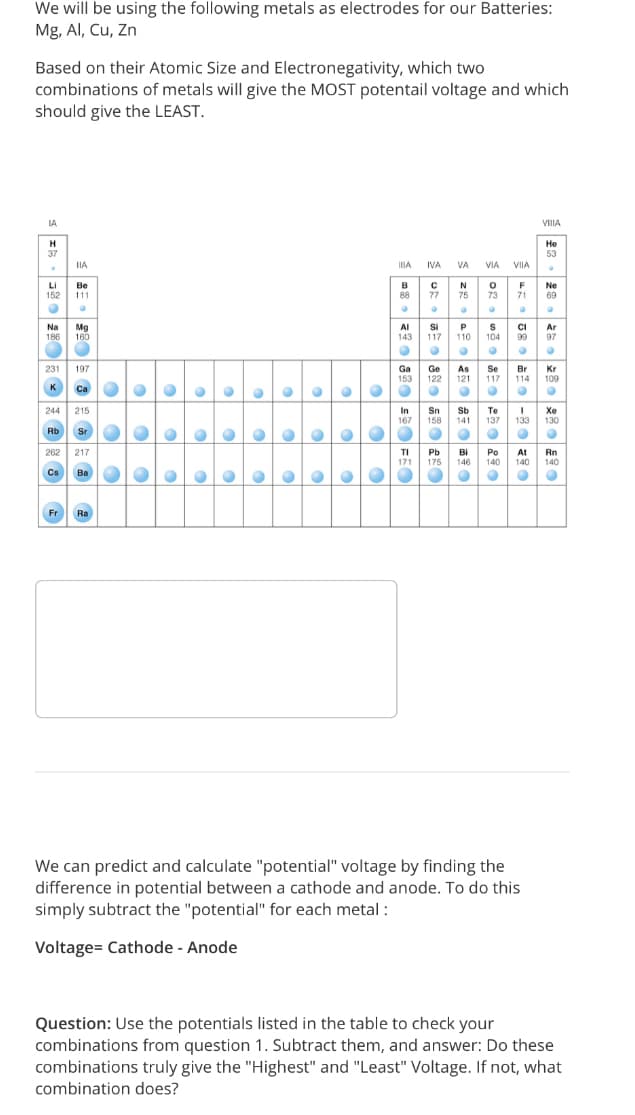
Transcribed Image Text:We will be using the following metals as electrodes for our Batteries:
Mg, Al, Cu, Zn
Based on their Atomic Size and Electronegativity, which two
combinations of metals will give the MOST potentail voltage and which
should give the LEAST.
IA
VIIIA
H
He
37
53
IIA
IA IVA
VA
VIA
VIIA
F
Li
152
Be
B
88
N
75
Ne
69
111
77
73
71
Si
117
Na
Mg
160
AI
Ar
186
143
110
104
99
97
Se
117
231
197
Ga
Ge
122
As
121
Br
114
Kr
109
153
K
Ca
Sn
158
244
215
In
167
Sb
Te
137
Xe
130
141
133
Rb
Sr
262
217
TI
Pb
175
Bi
146
Po
140
At
140
Rn
140
171
Cs Ba
Fr
Ra
We can predict and calculate "potential" voltage by finding the
difference in potential between a cathode and anode. To do this
simply subtract the "potential" for each metal :
Voltage= Cathode - Anode
Question: Use the potentials listed in the table to check your
combinations from question 1. Subtract them, and answer: Do these
combinations truly give the "Highest" and "Least" Voltage. If not, what
combination does?
O O ol
이 이 이
이 이 이
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning