2 a) Write the half-reaction for a hydrogen electrode in acidic solution when it is serving as the cathode of a voltaic cell. Define what is meant by the standard hydrogen electrode. What role does platinum play? b) For the half cells: Fe3+ + e-> Fe2+ E° = 0.77 V Cu2+ + 2e-> Cu E = 0.34 V Write the equation describing the reaction of an electrochemical cell comprised of the two half cells and calculate AG° for the cell. c)The standard reduction potentials for the following reactions are: 0.80 V Ag*(aq) + e- > Ag(s)
2 a) Write the half-reaction for a hydrogen electrode in acidic solution when it is serving as the cathode of a voltaic cell. Define what is meant by the standard hydrogen electrode. What role does platinum play? b) For the half cells: Fe3+ + e-> Fe2+ E° = 0.77 V Cu2+ + 2e-> Cu E = 0.34 V Write the equation describing the reaction of an electrochemical cell comprised of the two half cells and calculate AG° for the cell. c)The standard reduction potentials for the following reactions are: 0.80 V Ag*(aq) + e- > Ag(s)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 83AP
Related questions
Question
Please answer fast i will upvote
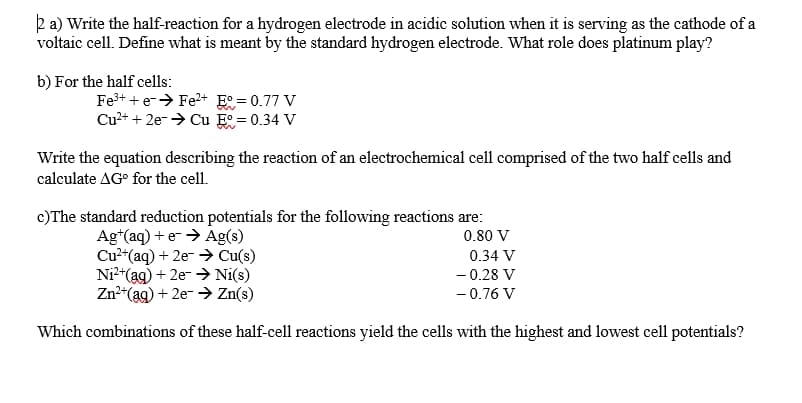
Transcribed Image Text:ka) Write the half-reaction for a hydrogen electrode in acidic solution when it is serving as the cathode of a
voltaic cell. Define what is meant by the standard hydrogen electrode. What role does platinum play?
b) For the half cells:
Fe3+ + e-> Fe2+ E° = 0.77 V
Cu2+ + 2e-→ Cu E = 0.34 V
Write the equation describing the reaction of an electrochemical cell comprised of the two half cells and
calculate AG° for the cell.
c)The standard reduction potentials for the following reactions are:
Ag*(aq) + e- > Ag(s)
Cu2"(aq) + 2e- > Cu(s)
Ni*(ag) + 2e- > Ni(s)
Zn*(ag) + 2e- > Zn(s)
0.80 V
0.34 V
- 0.28 V
- 0.76 V
Which combinations of these half-cell reactions yield the cells with the highest and lowest cell potentials?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning