What are each of the following observations an example of? Drag the appropriate items to their respective bins. If the tightly packed food is placed in the kitchen for a long time then you can smell the gas as it penetrates through the small holes in the plastic. Diffusion A balloon in a constant environment gets smaller and smaller over the course of a week as gas leaks through small holes in the rubber. When person applies perfume in one corner of the room you can smell its fragrance in another room. Effusion When a coworker microwaves popcorn, you can smell the vapors in your office 5 minutes later.
What are each of the following observations an example of? Drag the appropriate items to their respective bins. If the tightly packed food is placed in the kitchen for a long time then you can smell the gas as it penetrates through the small holes in the plastic. Diffusion A balloon in a constant environment gets smaller and smaller over the course of a week as gas leaks through small holes in the rubber. When person applies perfume in one corner of the room you can smell its fragrance in another room. Effusion When a coworker microwaves popcorn, you can smell the vapors in your office 5 minutes later.
Chapter5: Gases
Section: Chapter Questions
Problem 108E
Related questions
Question
100%
Pls help ASAP. Pls circle the final answer and place give proper units and decimals.
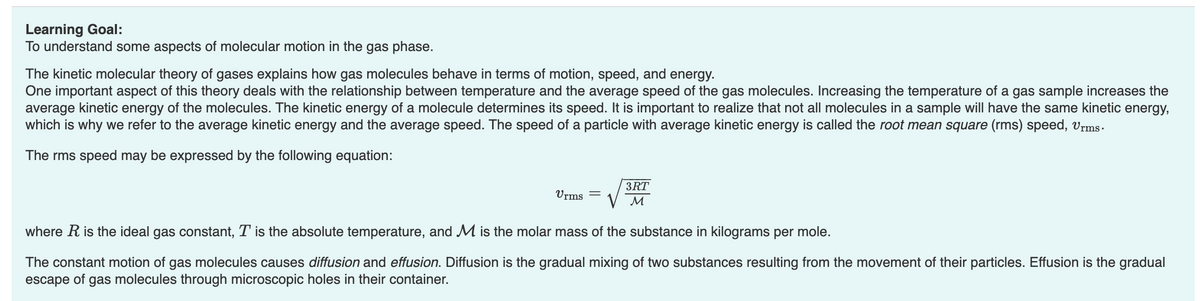
Transcribed Image Text:Learning Goal:
To understand some aspects of molecular motion in the gas phase.
The kinetic molecular theory of gases explains how gas molecules behave in terms of motion, speed, and energy.
One important aspect of this theory deals with the relationship between temperature and the average speed of the gas molecules. Increasing the temperature of a gas sample increases the
average kinetic energy of the molecules. The kinetic energy of a molecule determines its speed. It is important to realize that not all molecules in a sample will have the same kinetic energy,
which is why we refer to the average kinetic energy and the average speed. The speed of a particle with average kinetic energy is called the root mean square (rms) speed, Urms.
The rms speed may be expressed by the following equation:
Urms
3RT
M
where R is the ideal gas constant, T is the absolute temperature, and M is the molar mass of the substance in kilograms per mole.
The constant motion of gas molecules causes diffusion and effusion. Diffusion is the gradual mixing of two substances resulting from the movement of their particles. Effusion is the gradual
escape of gas molecules through microscopic holes in their container.
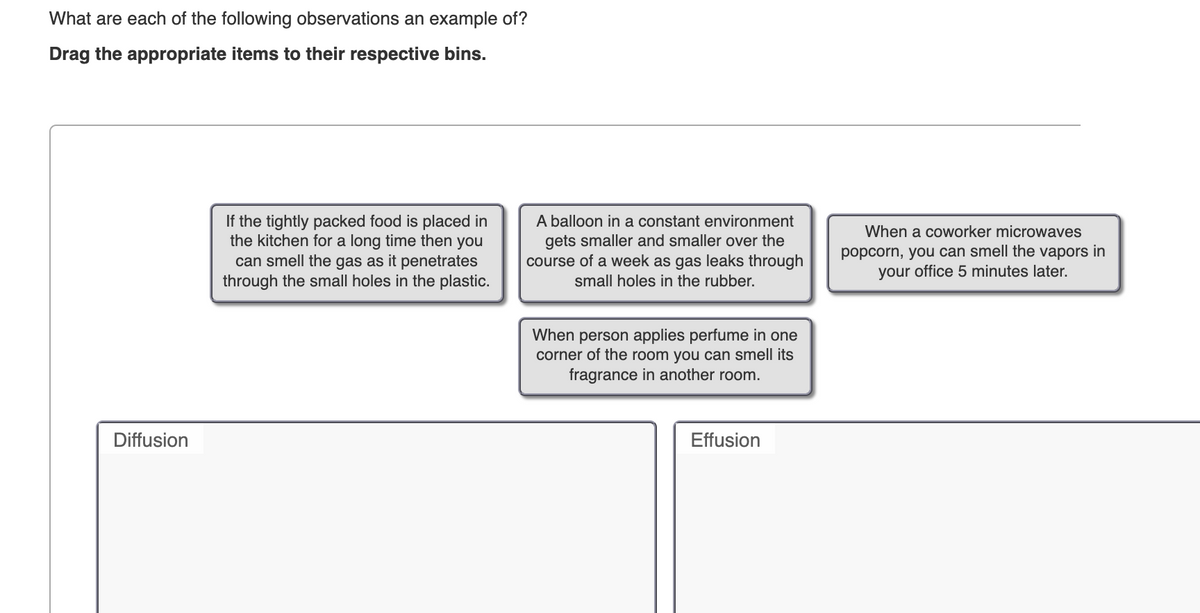
Transcribed Image Text:What are each of the following observations an example of?
Drag the appropriate items to their respective bins.
If the tightly packed food is placed in
the kitchen for a long time then you
can smell the gas as it penetrates
through the small holes in the plastic.
Diffusion
A balloon in a constant environment
gets smaller and smaller over the
course of a week as gas leaks through
small holes in the rubber.
When person applies perfume in one
corner of the room you can smell its
fragrance in another room.
Effusion
When a coworker microwaves
popcorn, you can smell the vapors in
your office 5 minutes later.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co