What atomic or hybrid orbitals make up the sigma bond between C, and O, in acetic acid, CH3COOH ? (C, is the second carbon in the structure as written.) orbital on C, + orbital on O What is the approximate C1-C2-O̟ bond angle ?
What atomic or hybrid orbitals make up the sigma bond between C, and O, in acetic acid, CH3COOH ? (C, is the second carbon in the structure as written.) orbital on C, + orbital on O What is the approximate C1-C2-O̟ bond angle ?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.100QE: The molecular orbital diagram of NO shown in Figure 10.47 also applies to the following species....
Related questions
Question
! ( answer with step by step explanation)
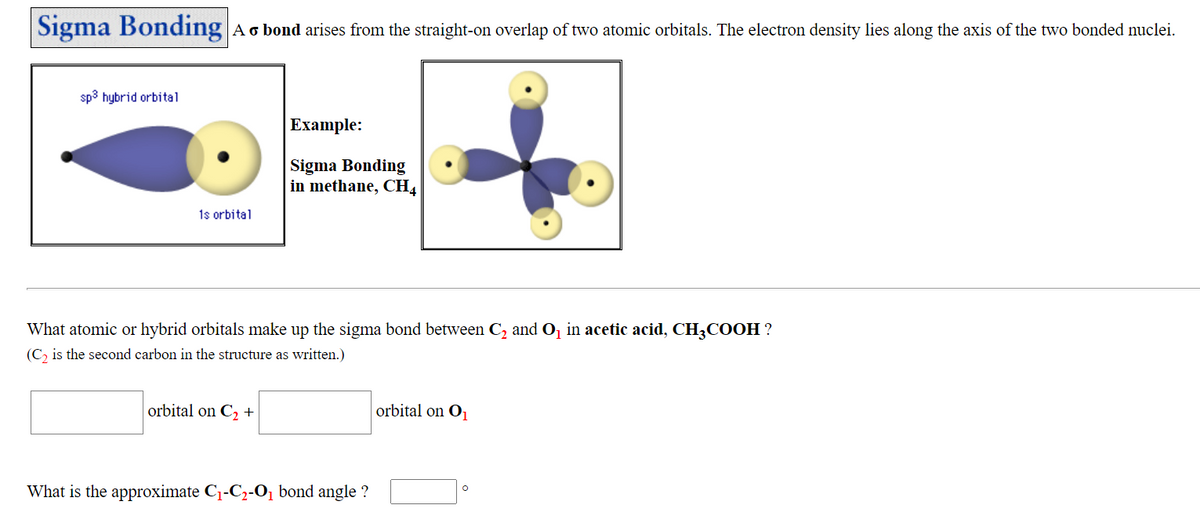
Transcribed Image Text:Sigma Bonding Ao bond arises from the straight-on overlap of two atomic orbitals. The electron density lies along the axis of the two bonded nuclei.
sp3 hybrid orbital
Example:
Sigma Bonding
in methane, CH4
1s orbital
What atomic or hybrid orbitals make up the sigma bond between C, and O, in acetic acid, CH3COOH ?
(C, is the second carbon in the structure as written.)
orbital on C2 +
orbital on O
What is the approximate C1-C2-O̟ bond angle ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning