What describes a solution that contains a system at equilibrium? One in which the color of the solution is changing slowly, or one in which the color is not changing. The following equilibrium is established when copper ions and bromide ions are placed in solution.
What describes a solution that contains a system at equilibrium? One in which the color of the solution is changing slowly, or one in which the color is not changing. The following equilibrium is established when copper ions and bromide ions are placed in solution.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter7: Covalent Bonding
Section: Chapter Questions
Problem 38QAP
Related questions
Question
- What describes a solution that contains a system at equilibrium? One in which the color of the solution is changing slowly, or one in which the color is not changing.
- The following equilibrium is established when copper ions and bromide ions are placed in solution.
The tube on the left contains only copper sulfate dissolved in solution. The tube on the right is the result of adding some potassium bromide (KBr) solution.
Given that the Cu(H2O)62+ ion is blue and that the CuBr42-ion is green;
a. What happened to the concentration of each of the ions when the KBr was added?
b. Explain why the solution changed in color.
c. Would the tube feel hot or cold when the KBr was added?

Transcribed Image Text:2-
ne i
ad
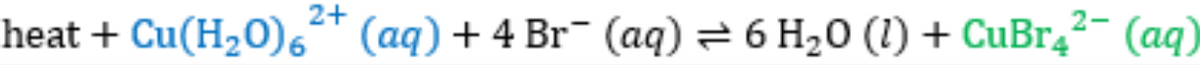
Transcribed Image Text:2+
heat + Cu(H20)6
2-
(aq) + 4 Br- (aq) = 6 H20 (1) + CuBr4
(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
