What impurity was likely removed during the recrystallization necessary and was it effective and why do you think so using the prcedure belwo and the reaction provided. PROCEDURE Synthesis of Dibenzalacetone (A) In this lab you will need to determine the amounts of the reactants you will use. We will use 0.0125 moles of acetone. You need to: (1) convert this into a volume in mL, (2) Calculate the number of moles of benzaldehyde needed based on the balanced chemical reaction, (3) Convert the number of moles of benzaldehyde into volume in mL. Your reagent table should reflect your calculated values. Mix the benzaldehyde and acetone based on the volumes from your prelab calculations in a testtube. Add 25 mL of 10% NaOH solution to a 125 mL Erlenmeyer flask along with a stir bar and 20 mL of ethanol. Make sure the temperature of the solution in the flask is below 25 C before proceeding. Add half of the solution from your test tube to the flask. Allow the solution to stir for 15 minutes and then add the remainder of the solution. Rinse the test tube with ethanol and allow the solution to stir for an additional 30 minutes. Workup (B) Collect the product with vacuum filtration. While in the Buckner funnel, wash the product three times with 50-mL of water. The vacuum should be stopped each time to effectively remove all the sodium hydroxide. The crystals will likely still fairly wet, attempt to dry to more by pressing a filter paper on top to absorb more water. Purify most of your product by recrystallizing from ethanol starting with ~ 4 mL for every gram. More ethanol maybe needed if your product is very wet. Collecting the Recrystallized M aterial (C) Vacuum filter your cold solution using pre-weighed filter paper, attempting to keep your stirbar in the Erlenmeyer flask. Wash the collected crystals (the “filter cake”) with an appropriate rinse solvent. Thendry your collected solid by allowing air to be pulled through the funnel for about fiveminutes or using the heatvac. Analysis of your Recrystallized M aterial (D) Once you’ve weighed your dry sample of recrystallized product, measure the melting points of both the crude and recrystallized samples. Analyze the purity with TLC against the starting material in excess.
What impurity was likely removed during the recrystallization necessary and was it effective and why do you think so using the prcedure belwo and the reaction provided. PROCEDURE Synthesis of Dibenzalacetone (A) In this lab you will need to determine the amounts of the reactants you will use. We will use 0.0125 moles of acetone. You need to: (1) convert this into a volume in mL, (2) Calculate the number of moles of benzaldehyde needed based on the balanced chemical reaction, (3) Convert the number of moles of benzaldehyde into volume in mL. Your reagent table should reflect your calculated values. Mix the benzaldehyde and acetone based on the volumes from your prelab calculations in a testtube. Add 25 mL of 10% NaOH solution to a 125 mL Erlenmeyer flask along with a stir bar and 20 mL of ethanol. Make sure the temperature of the solution in the flask is below 25 C before proceeding. Add half of the solution from your test tube to the flask. Allow the solution to stir for 15 minutes and then add the remainder of the solution. Rinse the test tube with ethanol and allow the solution to stir for an additional 30 minutes. Workup (B) Collect the product with vacuum filtration. While in the Buckner funnel, wash the product three times with 50-mL of water. The vacuum should be stopped each time to effectively remove all the sodium hydroxide. The crystals will likely still fairly wet, attempt to dry to more by pressing a filter paper on top to absorb more water. Purify most of your product by recrystallizing from ethanol starting with ~ 4 mL for every gram. More ethanol maybe needed if your product is very wet. Collecting the Recrystallized M aterial (C) Vacuum filter your cold solution using pre-weighed filter paper, attempting to keep your stirbar in the Erlenmeyer flask. Wash the collected crystals (the “filter cake”) with an appropriate rinse solvent. Thendry your collected solid by allowing air to be pulled through the funnel for about fiveminutes or using the heatvac. Analysis of your Recrystallized M aterial (D) Once you’ve weighed your dry sample of recrystallized product, measure the melting points of both the crude and recrystallized samples. Analyze the purity with TLC against the starting material in excess.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter11: Alkanes
Section: Chapter Questions
Problem 11.24P
Related questions
Question
What impurity was likely removed during the recrystallization necessary and
was it effective and why do you think so using the prcedure belwo and the reaction provided.
was it effective and why do you think so using the prcedure belwo and the reaction provided.
PROCEDURE
Synthesis of Dibenzalacetone (A)
In this lab you will need to determine the amounts of the reactants you will use. We will use 0.0125
moles of acetone. You need to: (1) convert this into a volume in mL, (2) Calculate the number of
moles of benzaldehyde needed based on the balancedchemical reaction , (3) Convert the number of
moles of benzaldehyde into volume in mL. Your reagent table should reflect your calculated values.
In this lab you will need to determine the amounts of the reactants you will use. We will use 0.0125
moles of acetone. You need to: (1) convert this into a volume in mL, (2) Calculate the number of
moles of benzaldehyde needed based on the balanced
moles of benzaldehyde into volume in mL. Your reagent table should reflect your calculated values.
Mix the benzaldehyde and acetone based on the volumes from your prelab
calculations in a testtube. Add 25 mL of 10% NaOH solution to a 125 mL
Erlenmeyer flask along with a stir bar and 20 mL of ethanol. Make sure the
temperature of the solution in the flask is below 25 C before proceeding. Add half of
the solution from your test tube to the flask. Allow the solution to stir for 15 minutes
and then add the remainder of the solution. Rinse the test tube with ethanol and allow
the solution to stir for an additional 30 minutes.
calculations in a testtube. Add 25 mL of 10% NaOH solution to a 125 mL
Erlenmeyer flask along with a stir bar and 20 mL of ethanol. Make sure the
temperature of the solution in the flask is below 25 C before proceeding. Add half of
the solution from your test tube to the flask. Allow the solution to stir for 15 minutes
and then add the remainder of the solution. Rinse the test tube with ethanol and allow
the solution to stir for an additional 30 minutes.
Workup (B)
Collect the product with vacuum filtration. While in the Buckner funnel, wash the
product three times with 50-mL of water. The vacuum should be stopped each time
to effectively remove all the sodium hydroxide. The crystals will likely still fairly wet,
attempt to dry to more by pressing a filter paper on top to absorb more water. Purify
most of your product by recrystallizing from ethanol starting with ~ 4 mL for every
gram. More ethanol maybe needed if your product is very wet.
product three times with 50-mL of water. The vacuum should be stopped each time
to effectively remove all the sodium hydroxide. The crystals will likely still fairly wet,
attempt to dry to more by pressing a filter paper on top to absorb more water. Purify
most of your product by recrystallizing from ethanol starting with ~ 4 mL for every
gram. More ethanol maybe needed if your product is very wet.
Collecting the Recrystallized M aterial (C) Vacuum filter your cold solution using pre-weighed filter paper, attempting to keep your stirbar in the Erlenmeyer flask. Wash the collected crystals (the “filter cake”) with an appropriate rinse solvent. Thendry your collected solid by allowing air to be pulled through the funnel for about fiveminutes or using the heatvac.
Analysis of your Recrystallized M aterial (D) Once you’ve weighed your dry sample of recrystallized product, measure the melting points of both the crude and recrystallized samples. Analyze the purity with TLC
against the starting material in excess.
against the starting material in excess.
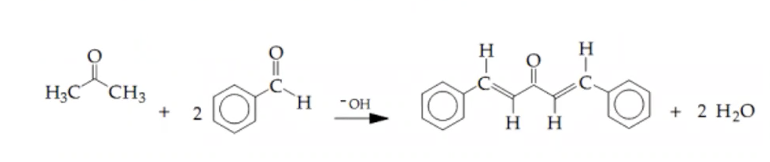
Transcribed Image Text:H
H
H3C CH3
+ 2
`H
-OH
+ 2 H2O
нн
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning