What is A GU per mole of dichromate ions for the reduction of dichromate ions, Cr 20 72-, to Cr 3+ by bromide ions, Br -, in acidic solution? (F = 96,500 J/V emol e ) Cr2072- + 14H* + 6Br → 3Br2(t) + 2Cr3+ + 7H20(E) O a. +145 kJ O b.-26.3 kJ OC -145 kJ O d. +26.3 kJ O e. -53.6 kJ
What is A GU per mole of dichromate ions for the reduction of dichromate ions, Cr 20 72-, to Cr 3+ by bromide ions, Br -, in acidic solution? (F = 96,500 J/V emol e ) Cr2072- + 14H* + 6Br → 3Br2(t) + 2Cr3+ + 7H20(E) O a. +145 kJ O b.-26.3 kJ OC -145 kJ O d. +26.3 kJ O e. -53.6 kJ
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter19: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 15E: The standard reduction potential for the reaction [Co( H 2 O)6]3+(aq)+e[CO( H 2 O)6]2+(aq) is about...
Related questions
Question
100%
What is ? Go per mole of dichromate ions for the reduction of dichromate ions, Cr2072, to Cr" by bromide ions, Br, in acidic solution? (Hint: Use the standard cell potential.) (a) +26.3 kJ (b) -145 k (c) +145 kJ (d) -26.3 kJ (e) -53.6 k.J
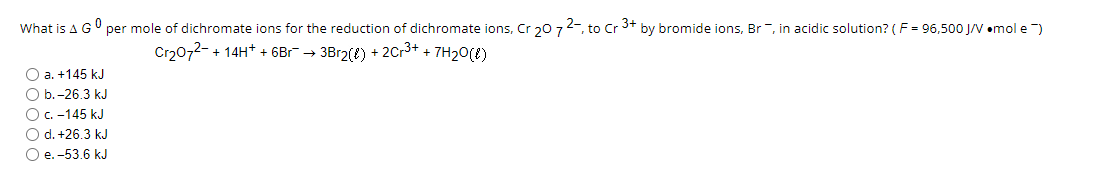
Transcribed Image Text:What is A GU per mole of dichromate ions for the reduction of dichromate ions, Cr 20 72-, to Cr 3+ by bromide ions, Br -, in acidic solution? (F = 96,500 J/V emol e )
Cr2072- + 14H* + 6Br → 3Br2(t) + 2Cr3+ + 7H20(E)
O a. +145 kJ
O b.-26.3 kJ
OC -145 kJ
O d. +26.3 kJ
O e. -53.6 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning