Calculate the standard reduction potential and the standard free energy change for the reaction: COQH; +2 cytochrome c-Fe + CoQ + 2 cytochrome c-Fe* + 2H* E, (CoQ/COQH;) =+ 0.06 volts and E. (cytochrome c- Fe"/ cytochrome c -Fe") = 0.25 volts (F = 96.48 kJ V' mol')
Calculate the standard reduction potential and the standard free energy change for the reaction: COQH; +2 cytochrome c-Fe + CoQ + 2 cytochrome c-Fe* + 2H* E, (CoQ/COQH;) =+ 0.06 volts and E. (cytochrome c- Fe"/ cytochrome c -Fe") = 0.25 volts (F = 96.48 kJ V' mol')
Chapter18: Introduction To Electrochemistry
Section: Chapter Questions
Problem 18.6QAP
Related questions
Question
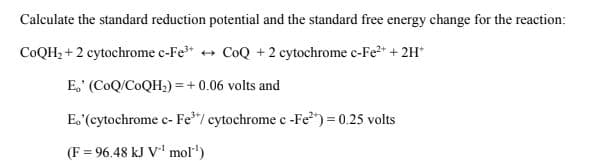
Transcribed Image Text:Calculate the standard reduction potential and the standard free energy change for the reaction:
COQH, + 2 cytochrome c-Fe* + CoQ +2 cytochrome c-Fe2+ + 2H*
E,' (CoQ/COQH;) = +0.06 volts and
E. (cytochrome c- Fe*"/ cytochrome c-Fe") = 0.25 volts
(F = 96.48 kJ V' mol")
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning