Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 1.2ACP
Related questions
Question
what is Hess’s Law? Explain which part(s) of the experiment (procedure and/or
treatment) it is applied.
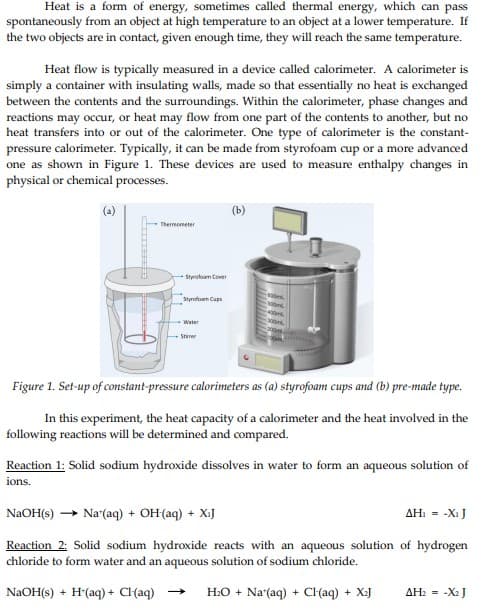
Transcribed Image Text:Heat is a form of energy, sometimes called thermal energy, which can pass
spontaneously from an object at high temperature to an object at a lower temperature. If
the two objects are in contact, given enough time, they will reach the same temperature.
Heat flow is typically measured in a device called calorimeter. A calorimeter is
simply a container with insulating walls, made so that essentially no heat is exchanged
between the contents and the surroundings. Within the calorimeter, phase changes and
reactions may occur, or heat may flow from one part of the contents to another, but no
heat transfers into or out of the calorimeter. One type of calorimeter is the constant-
pressure calorimeter. Typically, it can be made from styrofoam cup or a more advanced
one as shown in Figure 1. These devices are used to measure enthalpy changes in
physical or chemical processes.
Thermometer
Styrofoam Cover
Styrofoam Cups
Water
Stirrer
(b)
Figure 1. Set-up of constant-pressure calorimeters as (a) styrofoam cups and (b) pre-made type.
In this experiment, the heat capacity of a calorimeter and the heat involved in the
following reactions will be determined and compared.
Reaction 1: Solid sodium hydroxide dissolves in water to form an aqueous solution of
ions.
NaOH(s) Na (aq) + OH(aq) + X₁J
AH₁ = -X₁ J
Reaction 2: Solid sodium hydroxide reacts with an aqueous solution of hydrogen
chloride to form water and an aqueous solution of sodium chloride.
NaOH(s) + H(aq) + Cl(aq)
H₂O + Na'(aq) + Cl(aq) + X₂J
AH₂ = -X₂J
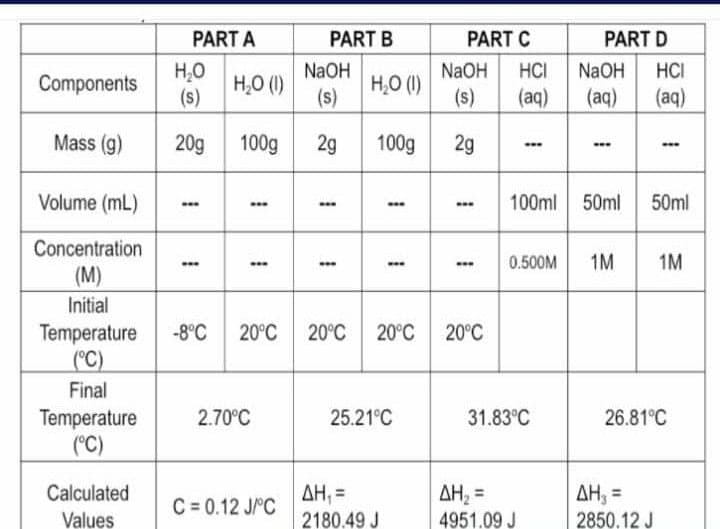
Transcribed Image Text:Components
Mass (g)
Volume (mL)
Concentration
(M)
Initial
Temperature
(°C)
Final
Temperature
(°C)
Calculated
Values
PART A
H₂O
(s)
20g 100g
1
www
H₂O (1)
.…
…….
2.70°C
PART B
C=0.12 J/°C
NaOH
(s)
2g
1
.….
H₂O (1)
100g
1
……w
ΔΗ, =
2180.49 J
-8°C 20°C 20°C 20°C 20°C
25.21°C
PART C
NaOH HCI
(s) (aq)
2g
www
www
0.500M
100ml 50ml
31.83°C
PART D
NaOH HCI
(aq) (aq)
AH₂ =
4951.09 J
-ww
www
50ml
1M 1M
26.81°C
AH₂ =
2850.12 J
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning