Chapter3: Atoms And Elements
Section: Chapter Questions
Problem 18E: Determine the number of protons and electrons in each of the following ions: a.Mg2+b.Se2-c.O2-d.Fe3+
Related questions
Question
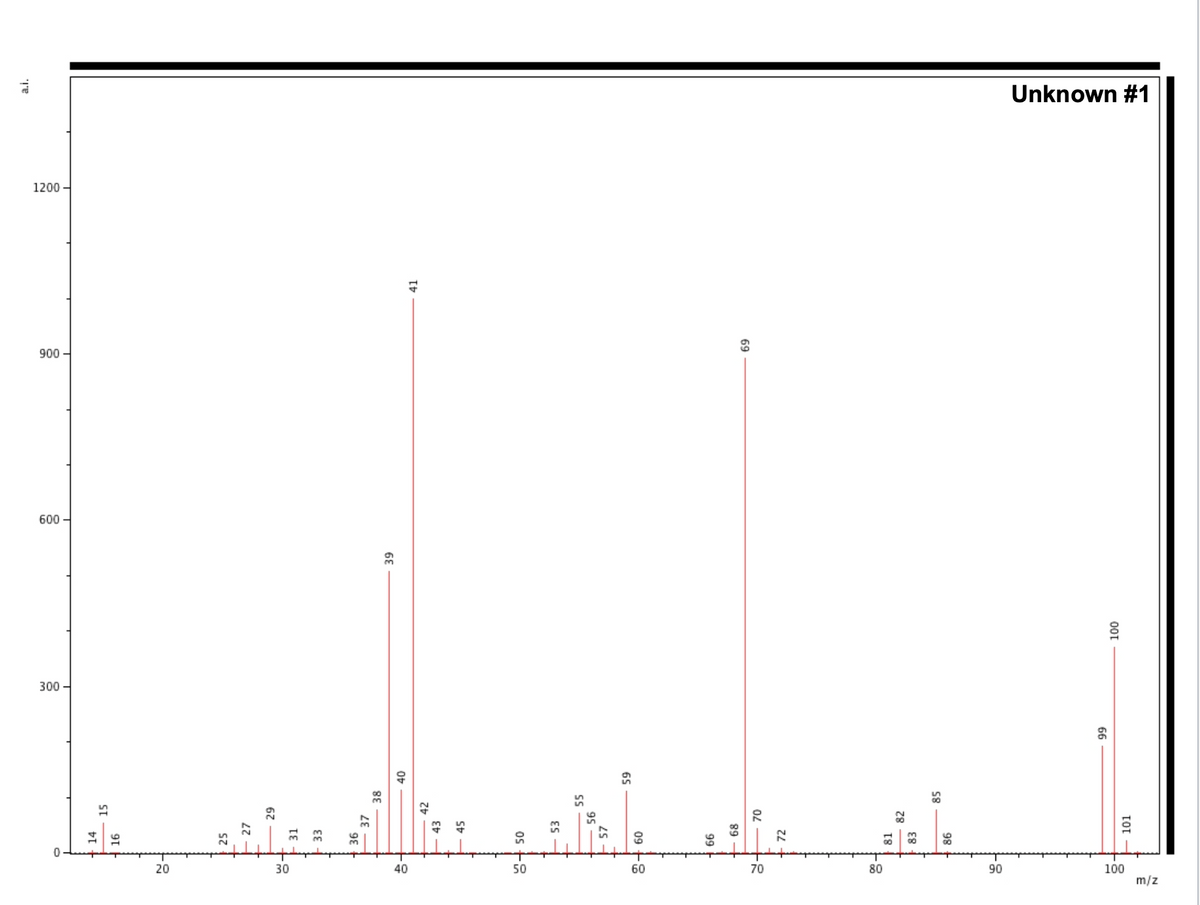
- What is M+, M+1 and M+2? Label these on spectrum.
- Calculate number carbons and nitrogen and using the equation. Start by assuming zero nitrogens; if the number of carbons does not come out to be a whole number, use the remainder to justify calculating number of nitrogens. This will not work out perfectly every time! Remember that these will be approximate depending on the signal intensity of the M+ peak and the presence of an [M-1]+ peak that can skew the isotope ratios. If this does not work out, use the value as an approximation to help guide your structure characterization.
- Calculate the number of oxygens using the equation. If there is no M+2 peak, state this. If the value is too low to be accurate, use it as an indicator for presence/absence of oxygen. This will not work out every time but will be used as a guide to determine chemical formula.
- Check the nitrogen rule – does it make sense with the M+ you observe and your calculations for N?
- Check whether fragments are even/odd and whether that matches with even/odd M+
- Determine chemical formula – does it add up to M+?
- Calculate degree of unsaturation – does this make sense with fragments observed?
- Draw structures for fragments on the spectrum – make sure they are charged and that you consider the scale is m/z, not just mass.
- At the higher mass end, include significant neutral losses, such as methyl, ethyl, OH, that are easily fragmented from the ion.
- Make sure you include the total predicted structure on your spectrum.
Transcribed Image Text:Unknown #1
1200
69
900
600
300 -
29
42
20
30
40
50
60
70
80
90
100
m/z
15
sz
E 27
67
31
33
98
F 37
38
99
89 -
72
81
- 82
58
98
66
F101
001
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning