For each of the ten spectra, you should determine which peaks are the most important for identification purposes, what their wavenumber values are, and what specific bonds and/or functional groups they indicate.
For each of the ten spectra, you should determine which peaks are the most important for identification purposes, what their wavenumber values are, and what specific bonds and/or functional groups they indicate.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter16: An Introduction To Infrared Spectrometry
Section: Chapter Questions
Problem 16.7QAP
Related questions
Question
For each of the ten spectra, you should determine which peaks are the most important for identification purposes, what their wavenumber values are, and what specific bonds and/or functional groups they indicate. Record those in the spaces under each spectrum. You don’t have to use each space. Then, from the list of possible compounds, identify what compound is responsible for each spectrum. Realize that these are all the real samples and there may be impurities present that cause weak peaks which would not normally be caused by the compound, such as the one at 3450 cm-1 in Spectrum 1.
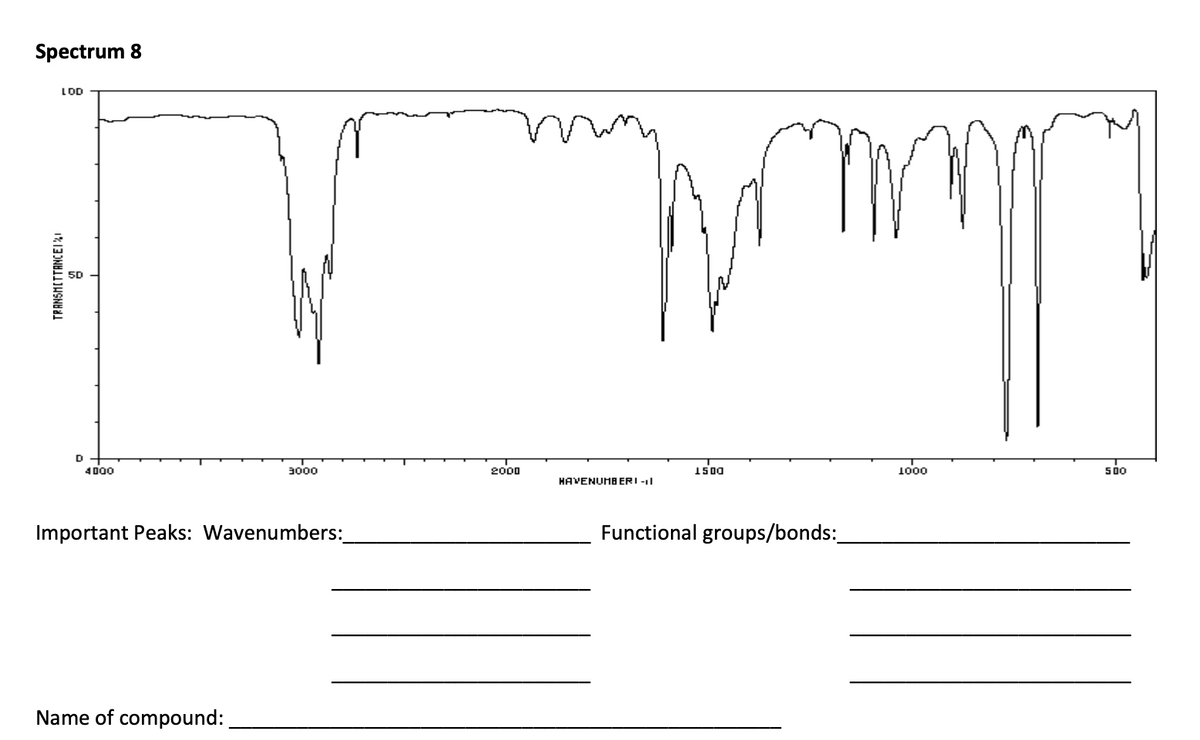
Transcribed Image Text:Spectrum 8
LOD
TRANSMITTANCE1%
D
4000
3000
Important Peaks: Wavenumbers:
Name of compound:
2000
HAVENUMBERI-I
1500
mapy
Functional groups/bonds:_
1000
500
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,