Biology 2e
2nd Edition
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:Matthew Douglas, Jung Choi, Mary Ann Clark
Chapter18: Evolution And The Origin Of Species
Section: Chapter Questions
Problem 13RQ: Which condition is the basis for a species to be re productively isolated from other members? It...
Related questions
Question
100%
What is the “cost of males” in outcrossing reproducing species compared to obligate self-fertilizer or asexually reproducing species?
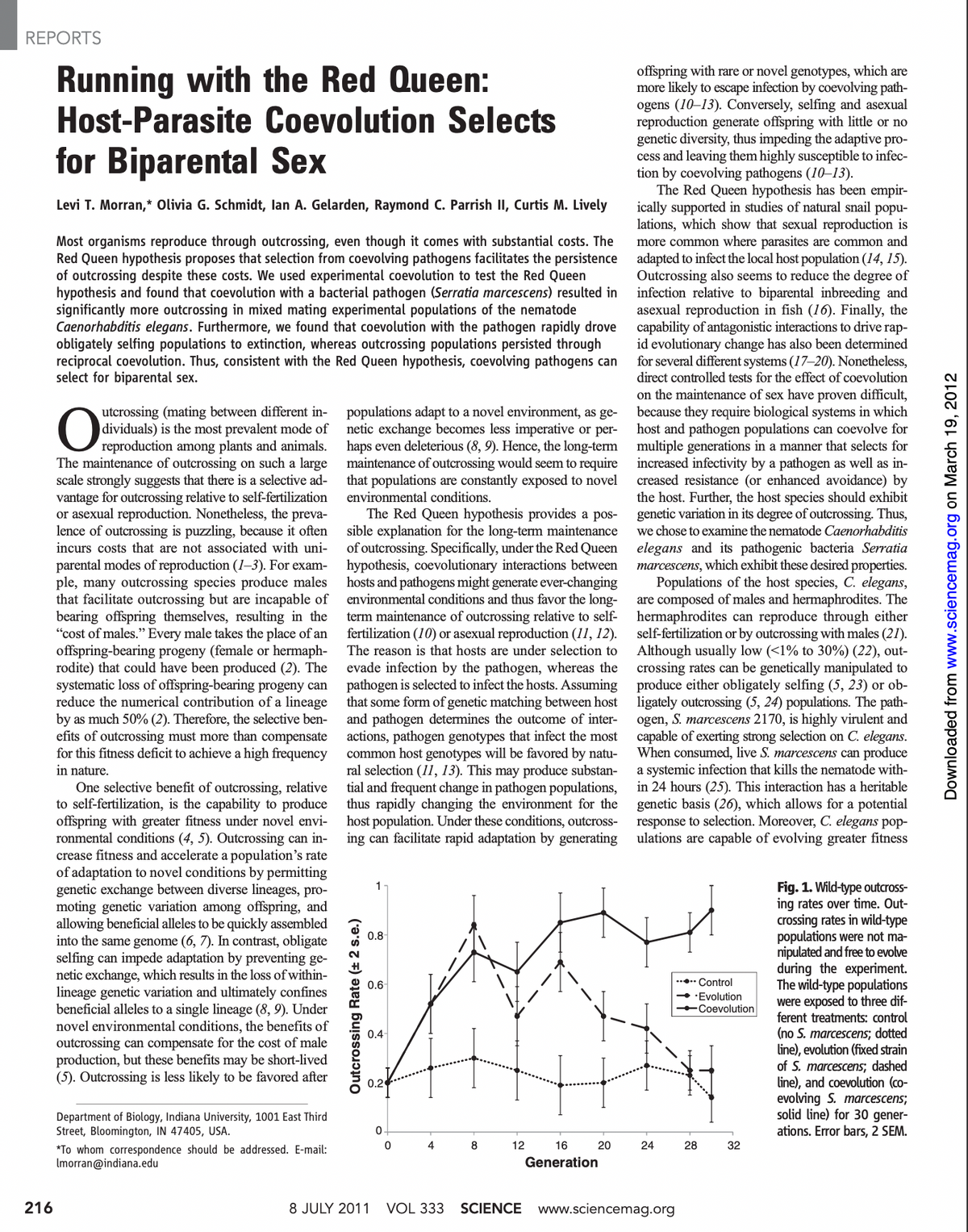
Transcribed Image Text:REPORTS
216
Running with the Red Queen:
Host-Parasite
for Biparental Sex
Levi T. Morran,* Olivia G. Schmidt, lan A. Gelarden, Raymond C. Parrish II, Curtis M. Lively
Coevolution Selects
Most organisms reproduce through outcrossing, even though it comes with substantial costs. The
Red Queen hypothesis proposes that selection from coevolving pathogens facilitates the persistence
of outcrossing despite these costs. We used experimental coevolution to test the Red Queen
hypothesis and found that coevolution with a bacterial pathogen (Serratia marcescens) resulted in
significantly more outcrossing in mixed mating experimental populations of the nematode
Caenorhabditis elegans. Furthermore, we found that coevolution with the pathogen rapidly drove
obligately selfing populations to extinction, whereas outcrossing populations persisted through
reciprocal coevolution. Thus, consistent with the Red Queen hypothesis, coevolving pathogens can
select for biparental sex.
O
utcrossing (mating between different in-
dividuals) is the most prevalent mode of
reproduction among plants and animals.
The maintenance of outcrossing on such a large
scale strongly suggests that there is a selective ad-
vantage for outcrossing relative to self-fertilization
or asexual reproduction. Nonetheless, the preva-
lence of outcrossing is puzzling, because it often
incurs costs that are not associated with uni-
parental modes of reproduction (1-3). For exam-
ple, many outcrossing species produce males
that facilitate outcrossing but are incapable of
bearing offspring themselves, resulting in the
"cost of males." Every male takes the place of an
offspring-bearing progeny (female or hermaph-
rodite) that could have been produced (2). The
systematic loss of offspring-bearing progeny can
reduce the numerical contribution of a lineage
by as much 50% (2). Therefore, the selective ben-
efits of outcrossing must more than compensate
for this fitness deficit to achieve a high frequency
in nature.
One selective benefit of outcrossing, relative
to self-fertilization, is the capability to produce
offspring with greater fitness under novel envi-
ronmental conditions (4, 5). Outcrossing can in-
crease fitness and accelerate a population's rate
of adaptation to novel conditions by permitting
genetic exchange between diverse lineages, pro-
moting genetic variation among offspring, and
allowing beneficial alleles to be quickly assembled
into the same genome (6, 7). In contrast, obligate
selfing can impede adaptation by preventing ge-
netic exchange, which results in the loss of within-
lineage genetic variation and ultimately confines
beneficial alleles to a single lineage (8, 9). Under
novel environmental conditions, the benefits of
outcrossing can compensate for the cost of male
production, but these benefits may be short-lived
(5). Outcrossing is less likely to be favored after
Department of Biology, Indiana University, 1001 East Third
Street, Bloomington, IN 47405, USA.
*To whom correspondence should be addressed. E-mail:
Imorran@indiana.edu
populations adapt to a novel environment, as ge-
netic exchange becomes less imperative or per-
haps even deleterious (8, 9). Hence, the long-term
maintenance of outcrossing would seem to require
that populations are constantly exposed to novel
environmental conditions.
The Red Queen hypothesis provides a pos-
sible explanation for the long-term maintenance
of outcrossing. Specifically, under the Red Queen
hypothesis, coevolutionary interactions between
hosts and pathogens might generate ever-changing
environmental conditions and thus favor the long-
term maintenance of outcrossing relative to self-
fertilization (10) or asexual reproduction (11, 12).
The reason is that hosts are under selection to
evade infection by the pathogen, whereas the
pathogen is selected to infect the hosts. Assuming
that some form of genetic matching between host
and pathogen determines the outcome of inter-
actions, pathogen genotypes that infect the most
common host genotypes will be favored by natu-
ral selection (11, 13). This may produce substan-
tial and frequent change in pathogen populations,
thus rapidly changing the environment for the
host population. Under these conditions, outcross-
ing can facilitate rapid adaptation by generating
Outcrossing Rate (+ 2 s.e.)
1
0.8-
0.6
0.4
0.2
8 JULY 2011
0
0
4
VOL 333
8
12
16
Generation
20
offspring with rare or novel genotypes, which are
more likely to escape infection by coevolving path-
ogens (10-13). Conversely, selfing and asexual
reproduction generate offspring with little or no
genetic diversity, thus impeding the adaptive pro-
cess and leaving them highly susceptible to infec-
tion by coevolving pathogens (10-13).
The Red Queen hypothesis has been empir-
ically supported in studies of natural snail popu-
lations, which show that sexual reproduction is
more common where parasites are common and
adapted to infect the local host population (14, 15).
Outcrossing also seems to reduce the degree of
infection relative to biparental inbreeding and
asexual reproduction in fish (16). Finally, the
capability of antagonistic interactions to drive rap-
id evolutionary change has also been determined
for several different systems (17-20). Nonetheless,
direct controlled tests for the effect of coevolution
on the maintenance of sex have proven difficult,
because they require biological systems in which
host and pathogen populations can coevolve for
multiple generations in a manner that selects for
increased infectivity by a pathogen as well as in-
creased resistance (or enhanced avoidance) by
the host. Further, the host species should exhibit
genetic variation in its degree of outcrossing. Thus,
we chose to examine the nematode Caenorhabditis
elegans and its pathogenic bacteria Serratia
marcescens, which exhibit these desired properties.
Populations of the host species, C. elegans,
are composed of males and hermaphrodites. The
hermaphrodites can reproduce through either
self-fertilization or by outcrossing with males (21).
Although usually low (<1% to 30%) (22), out-
crossing rates can be genetically manipulated to
produce either obligately selfing (5, 23) or ob-
ligately outcrossing (5, 24) populations. The path-
ogen, S. marcescens 2170, is highly virulent and
capable of exerting strong selection on C. elegans.
When consumed, live S. marcescens can produce
a systemic infection that kills the nematode with-
in 24 hours (25). This interaction has a heritable
genetic basis (26), which allows for a potential
response to selection. Moreover, C. elegans pop-
ulations are capable of evolving greater fitness
24
SCIENCE www.sciencemag.org
...Control
→Evolution
Coevolution
28
32
Fig. 1. Wild-type outcross-
ing rates over time. Out-
crossing rates in wild-type
populations were not ma-
nipulated and free to evolve
during the experiment.
The wild-type populations
were exposed to three dif-
ferent treatments: control
(no S. marcescens; dotted
line), evolution (fixed strain
of S. marcescens; dashed
line), and coevolution (co-
evolving S. marcescens;
solid line) for 30 gener-
ations. Error bars, 2 SEM.
Downloaded from www.sciencemag.org on March 19, 2012
![in response to S. marcescens exposure (5), and
S. marcescens can evolve greater infectivity when
successful infection of C. elegans is its only means
of proliferation. Selection for increased infectiv-
ity can be imposed by propagating only those
bacterial cells that have been harvested from the
carcasses of hosts, which were killed by the bacte-
ria within 24 hours of exposure. Therefore, the
C. elegans/S. marcescens system can be used to
generate antagonistic coevolution when a host pop-
ulation and a pathogen population are repeatedly
passaged under selection together, thus permitting
a direct test of the Red Queen hypothesis.
We used experimental coevolution in the
C. elegans/S. marcescens system to test the pre-
diction that antagonistic coevolution between
host and pathogen populations can maintain high
levels of outcrossing despite the inherent cost of
males. We used obligately selfing, wild-type, and
obligately outcrossing populations of C. elegans
with a CB4856 genetic background (5). Where-
as the reproductive modes of the obligately self-
ing and obligately outcrossing populations are
genetically fixed, the wild-type populations can
Fig. 2. Coevolutionary dynamics of
hosts and pathogens. We exposed
hosts evolved under the coevolution
treatment and their ancestral popu-
lations (before coevolution) to three
pathogen populations: (i) an ancestor
strain (ancestral to all S. marcescens
used in this study), (ii) a noncoevolv-
ing strain (evolved without selection),
and (iii) their respective coevolving
strain (coevolving with the host pop-
ulation). We evaluated host mortal-
ity after 24 hours of exposure to the
pathogens and present the means
across the replicate host populations.
(A) Three obligately selfing C. elegans
populations persisted beyond 10 host
generations in the coevolution treat-
ment. These populations were assayed
before extinction. (B) All five wild-
type C. elegans populations in the
coevolution treatment and their an-
cestors were assayed at the endpoint
of the experiment (30 host gener-
ations). (C) All five obligately out-
crossing C. elegans populations in the
coevolution treatment and their an-
cestors were assayed at the endpoint
of the experiment. Error bars, 2 SEM.
Host Mortality Rate at 24 Hours of Exposure (+ 2 s.e.)
0.8, A
0.6
0.4+
0.2-
0
0.8, B
0.6-
0.4-
0.2
0
0.8, C
0.6
0.4
0.2
0
reproduce by either selfing or outcrossing [the
baseline outcrossing rate is ~20 to 30% (5)], and
the rate of outcrossing can respond to selection
(5). Before the experiment, we mutagenized five
independent replicate populations of each mating
type (obligate selfing, wild-type, and obligate out-
crossing) by exposing them to ethyl methane-
sulfonate (EMS) to infuse novel genetic variation
in each population. The five replicate populations
were then passaged under three different para-
site treatments (table S1): (i) control (no exposure
to S. marcescens), (ii) evolution (repeated expo-
sure to a fixed, nonevolving strain of S. marcescens),
and (iii) coevolution. The coevolution treatment in-
volved repeated exposure (30 host generations) to
a potentially coevolving population of S. marcescens,
which was under selection for increased infectiv-
ity. S. marcescens Sm2170 served as the ancestral
strain in the coevolution treatment, as well as the
fixed strain in the evolution treatment.
The results were consistent with the Red
Queen hypothesis. In the coevolution treatment,
all of the obligately selfing populations became
extinct within 20 generations (fig. S1). However,
S. marcescens
Ancestor
Non-coevolving
Coevolving
Obligately Selfing C. elegans
b
a
Ancestral Populations
Generation 10
"Coevolution" Populations
Wildtype (Mixed Mating) C. elegans
g
Ancestral Populations
Generation 30
"Coevolution" Populations
Obligately Outcrossing C. elegans
m
Ancestral Populations
n
Generation 30
"Coevolution" Populations
REPORTS
none of the obligately selfing populations went
extinct in either the evolution treatment or in the
control treatment. In addition, all of the obligately
outcrossing and wild-type populations persisted
throughout the experiment in all three treatment
types (fig. S1). Thus, extinction was only ob-
served in obligately selfing hosts when confronted
with coevolving pathogens.
We also found that the presence of coevolving
S. marcescens selected for and maintained high
levels of outcrossing in wild-type C. elegans pop-
ulations (Fig. 1). Over the first eight generations
of the experiment, outcrossing rates increased
from 20% to more than 70% in both the evo-
lution and coevolution treatments (Fig. 1) (F2,11=
8.26; P = 0.006). However, the wild-type popu-
lations consistently exposed to a fixed population
of S. marcescens (evolution treatment) exhibited
a steady decline in outcrossing rates after this ini-
tial increase, eventually returning to control levels
of outcrossing (Fig. 1), as previously observed (5).
In contrast, populations in the coevolution treat-
ment consistently maintained high levels of out-
crossing throughout the experiment, relative to
the control treatment (Fig. 1) (F1,12 = 209.5; P<
0.0001). Coevolution with S. marcescens, there-
fore, favored the evolution and long-term main-
tenance of higher rates of outcrossing.
As also predicted by the Red Queen hypoth-
esis, outcrossing hosts adapted to changes in the
pathogen population, whereas selfing apparently
prevented an adaptive counter-response. The an-
cestral strain of the obligately selfing hosts suffered
higher mortality rates when exposed to bacteria
from the coevolution treatment than when ex-
posed to either the ancestral bacteria (Fig. 2A)
(c> a: F1,71 = 21.2; P<0.0001) or to the nonco-
evolving control bacteria (Fig. 2A) (c>b: F1,71 =
31.9; P<0.0001). Therefore, the bacteria in the
coevolution treatment evolved greater infectivity
in response to selection. Further, the obligately
selfing hosts did not adapt to the evolution of
increased infectivity in the bacteria, because
the bacteria from the coevolution treatment in-
duced greater levels of mortality against the worms
after 10 generations of coevolution than against
the ancestral hosts (Fig. 2A) (f>c: F1,71 = 69.2;
P<0.0001). Finally, an increase in mortality
by more than a factor of 3 was observed in the
obligately selfing hosts in only 10 generations
(Fig. 2A) (f> a: F1,71 = 173.7; P < 0.0001),
which could explain why these hosts were driven
to extinction.
The pathogens that coevolved with the wild-
type and obligate outcrossing populations also
evolved greater infectivity (Fig. 2, B and C) (i > h:
F1,104 69.5; P<0.0001; i >g: F1,104 = 32.9; P<
0.0001; on: F1,60 = 141.1; P<0.0001; o > m:
F1,60 50.9; P<0.0001). However, the wild-type
and obligately outcrossing populations adapted
to the changes in their respective coevolving path-
ogen populations. Specifically, both the wild-type
and obligately outcrossing populations exhibited
lower mortality rates against the pathogens with
which they were currently evolving than did their
www.sciencemag.org SCIENCE VOL 333 8 JULY 2011
217
Downloaded from www.sciencemag.org on March 19, 2012
REPORTS
218
ancestors (Fig. 2, B and C) (i >l: F1,104=27.9; P<
0.0001; o>r: F1,60 = 166.2; P<0.0001), thus
indicating reciprocal coevolution in the outcross-
ing host populations. Whereas the obligate selfing
populations in the coevolution treatment became
more infected over time (Fig. 2A), the wild-type
populations maintained the same level of infec-
tivity over the course of the experiment (Fig. 2B)
(g=l: F1,104 0.35; P=0.554), while the obligate
outcrossing populations were significantly less
infected at the end of the experiment relative to
the beginning (Fig. 2C) (m>r: F1,60 = 33.1; P<
0.0001). Coupled with the maintenance of high
outcrossing rates in the coevolving wild-type
populations (Fig. 1), these results demonstrate the
ability of antagonistic coevolution to continually
generate novel environmental conditions under
which outcrossing is favored and populations per-
sist when interacting with a virulent pathogen.
A recent host/pathogen coevolution study in
C. elegans further supports the conclusion that
low levels of outcrossing impede the rate of
adaptive evolution. The C. elegans hosts in this
previous study appear to have primarily repro-
duced via self-fertilization and did not evolve
significantly greater resistance to a coevolving
pathogen over 48 generations of selection (27).
Contrary to our study, however, greater out-
crossing rates did not evolve in these mixed-
mating populations in response to the pathogen.
It may be that higher levels of genetic variation
and/or a greater level of pathogen virulence in
our study account for the difference in outcomes.
In summary, we found that obligately selfing
lineages were driven to extinction when con-
fronted with a coevolving parasite. These results
are consistent with the macroevolutionary aspects
of the Red Queen hypothesis, as originally formu-
lated by Van Valen (28). We also found that the
presence of a coevolving pathogen selected for and
maintained high levels of outcrossing in mixed-
mating populations, whereas elevated levels of
outcrossing were not maintained in populations
where the pathogen was not coevolving. These
results are consistent with the microevolutionary
predictions of the Red Queen. Taken together, the
results demonstrate that sex can facilitate adap-
tation to novel environments, but the long-term
maintenance of sex requires that the novelty does
not wear off.
References and Notes
1. G. C. Williams, Sex and Evolution (Princeton University
Press, Princeton, NJ, 1975).
2. J. Maynard Smith, The Evolution of Sex (Cambridge
University Press, Cambridge, UK, 1978).
3. G. Bell, The Masterpiece of Nature: The Evolution and
Genetics of Sexuality (University of California Press,
Berkeley, CA, 1982).
4. G. L. Stebbins, Am. Nat. 91, 337 (1957).
5. L. T. Morran, M. D. Parmenter, P. C. Phillips, Nature 462,
350 (2009).
ature blood cell lineages are generated
from a network of hierarchically dis-
tinct progenitors that arise from self-
renewing hematopoietic stem cells (HSCs). The
extensive regenerative potential of HSCs makes
them attractive targets for cellular and genetic
6. H. J. Muller, Am. Nat. 66, 118 (1932).
7. R. A. Fisher, The Genetical Theory of Natural Selection
(Clarendon Press, Oxford, 1930).
8. R. Lande, D. W. Schemske, Evolution 39, 24 (1985).
9. D. Charlesworth, B. Charlesworth, Annu. Rev. Ecol. Syst.
18, 237 (1987).
10. A. F. Agrawal, C. M. Lively, Evolution 55, 869 (2001).
11. J. Jaenike, Evol. Theory 3, 191 (1978).
12. W. D. Hamilton, Oikos 35, 282 (1980).
13. W. Hamilton, R. Axelrod, R. Tanese, Proc
Sci. U.S.A. 87, 3566 (1990).
14. C. M. Lively, Nature 328, 519 (1987).
Isolation of Single Human Hematopoietic
Stem Cells Capable of Long-Term
Multilineage Engraftment
Faiyaz Notta,¹,²* Sergei Doulatov, ¹,2* Elisa Laurenti, ¹,² Armando Poeppl,¹
Igor Jurisica,3,4 John E. Dick¹, ²+
Acad.
Lifelong blood cell production is dependent on rare hematopoietic stem cells (HSCs) to
perpetually replenish mature cells via a series of lineage-restricted intermediates. Investigating
the molecular state of HSCs is contingent on the ability to purify HSCs away from transiently
engrafting cells. We demonstrated that human HSCs remain infrequent, using current purification
strategies based on Thy1 (CD90) expression. By tracking the expression of several adhesion
molecules in HSC-enriched subsets, we revealed CD49f as a specific HSC marker. Single CD49f+
cells were highly efficient in generating long-term multilineage grafts, and the loss of CD49f
expression identified transiently engrafting multipotent progenitors (MPPs). The demarcation of
human HSCs and MPPS will enable the investigation of the molecular determinants of HSCs,
with a goal of developing stem cell-based therapeutics.
therapies. The molecular regulation of specific
HSC properties such as long-term self-renewal is
beginning to be elucidated for murine HSCs (1).
However the biology of human HSCs remains
poorly understood because of their rarity and the
lack of methods to segregate HSCs from multip-
8 JULY 2011 VOL 333
15. K. C. King, L. F. Delph, J. Jokela, C. M. Lively, Curr. Biol.
19, 1438 (2009).
16. C. M. Lively, C. Craddock, R. C. Vrijenhoek, Nature 344,
864 (1990).
17. E. Decaestecker et al., Nature 450, 870 (2007).
18. B. Koskella, C. M. Lively, Evolution 63, 2213 (2009).
19. J. Jokela, M. F. Dybdahl, C. M. Lively, Am. Nat. 174
(suppl. 1), S43 (2009).
20. S. Paterson et al., Nature 464, 275 (2010).
21. S. Brenner, Genetics 77, 71 (1974).
22. H. Teotónio, D. Manoel, P. C. Phillips, Evolution 60, 1300
(2006).
23. L. M. Miller, J. D. Plenefisch, L. P. Casson, B. J. Meyer,
Cell 55, 167 (1988).
24. T. Schedl, J. Kimble, Genetics 119, 43 (1988).
25. C. L. Kurz et al., EMBO J. 22, 1451 (2003).
26. G. V. Mallo et al., Curr. Biol. 12, 1209 (2002).
27. R. D. Schulte, C. Makus, B. Hasert, N. K. Michiels,
H. Schulenburg, Proc. Natl. Acad. Sci. U.S.A. 107, 7359
(2010).
28. L. Van Valen, Evol. Theory 1, 1 (1973).
Acknowledgments: We thank H. Hundley and R. Matteson
for logistical assistance. We also thank F. Bashey,
L. Delph, P. Phillips, M. Parmenter, the Lively and Hall
laboratories, and two reviewers for helpful comments
and discussion, as well as the Wissenschaftskolleg zu
Berlin for a fellowship to C.M.L. during the preparation
of the manuscript. Funding was provided by the NSF
(DEB-0640639 to C.M.L) and the NIH (1F32GM096482-01
to L.T.M). Nematode strains were provided by the
Caenorhabditis Genetics Center, which is funded by the
NIH National Center for Research Resources (NCRR). Data
deposited at Dryad, 10.5061/dryad.c0q0h.
Supporting Online Material
www.sciencemag.org/cgi/content/full/333/6039/216/DC1
Materials and Methods
Fig. S1
Table S1
References 29 to 31
31 March 2011; accepted 24 May 2011
10.1126/science.1206360
otent progenitors (MPPs) to obtain pure popula-
tions for biological and molecular analysis.
The bulk of HSCs are CD34+, as evidenced
by human transplantation and xenograft re-
population assays; however, most CD34+ cells
are lineage-restricted progenitors and HSCs re-
main rare. HSCs can be enriched further on the
basis of CD45RA (2), Thy1 (3-5), and CD38
(6, 7) expression. Loss of Thyl expression in the
CD34 CD38 CD45RA compartment of lineage-
depleted cord blood (CB) was recently proposed
to be sufficient to separate HSCs from MPPS
(5). However, more than a third of Thy1 primary
recipients gave rise to engraftment in secondary
animals, raising uncertainty about whether Thyl
can absolutely segregate HSCs from MPPs. To
¹Division of Stem Cell and Developmental Biology, Campbell
Family Institute for Cancer Research/Ontario Cancer Institute,
Toronto, Ontario, Canada. ²Department of Molecular Genetics,
University of Toronto, Toronto, Ontario, Canada. ³Ontario
Cancer Institute and Campbell Family Institute for Cancer
Research, Toronto, Ontario, Canada. Departments of Com-
puter Science and Medical Biophysics, University of Toronto,
Toronto, Ontario, Canada.
*These authors contributed equally to this work.
To whom correspondence should be addressed. Toronto
Medical Discovery Tower, Room 8-301, 101 College Street,
Toronto, Canada M5G 1L7. E-mail: jdick@uhnres.utoronto.ca
SCIENCE www.sciencemag.org](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7a745978-2d4b-4e83-9772-d1b742bc80a7%2F5ebe9c5f-37ec-4c8a-87a7-1da3902de1cc%2Fefhawzt_processed.png&w=3840&q=75)
Transcribed Image Text:in response to S. marcescens exposure (5), and
S. marcescens can evolve greater infectivity when
successful infection of C. elegans is its only means
of proliferation. Selection for increased infectiv-
ity can be imposed by propagating only those
bacterial cells that have been harvested from the
carcasses of hosts, which were killed by the bacte-
ria within 24 hours of exposure. Therefore, the
C. elegans/S. marcescens system can be used to
generate antagonistic coevolution when a host pop-
ulation and a pathogen population are repeatedly
passaged under selection together, thus permitting
a direct test of the Red Queen hypothesis.
We used experimental coevolution in the
C. elegans/S. marcescens system to test the pre-
diction that antagonistic coevolution between
host and pathogen populations can maintain high
levels of outcrossing despite the inherent cost of
males. We used obligately selfing, wild-type, and
obligately outcrossing populations of C. elegans
with a CB4856 genetic background (5). Where-
as the reproductive modes of the obligately self-
ing and obligately outcrossing populations are
genetically fixed, the wild-type populations can
Fig. 2. Coevolutionary dynamics of
hosts and pathogens. We exposed
hosts evolved under the coevolution
treatment and their ancestral popu-
lations (before coevolution) to three
pathogen populations: (i) an ancestor
strain (ancestral to all S. marcescens
used in this study), (ii) a noncoevolv-
ing strain (evolved without selection),
and (iii) their respective coevolving
strain (coevolving with the host pop-
ulation). We evaluated host mortal-
ity after 24 hours of exposure to the
pathogens and present the means
across the replicate host populations.
(A) Three obligately selfing C. elegans
populations persisted beyond 10 host
generations in the coevolution treat-
ment. These populations were assayed
before extinction. (B) All five wild-
type C. elegans populations in the
coevolution treatment and their an-
cestors were assayed at the endpoint
of the experiment (30 host gener-
ations). (C) All five obligately out-
crossing C. elegans populations in the
coevolution treatment and their an-
cestors were assayed at the endpoint
of the experiment. Error bars, 2 SEM.
Host Mortality Rate at 24 Hours of Exposure (+ 2 s.e.)
0.8, A
0.6
0.4+
0.2-
0
0.8, B
0.6-
0.4-
0.2
0
0.8, C
0.6
0.4
0.2
0
reproduce by either selfing or outcrossing [the
baseline outcrossing rate is ~20 to 30% (5)], and
the rate of outcrossing can respond to selection
(5). Before the experiment, we mutagenized five
independent replicate populations of each mating
type (obligate selfing, wild-type, and obligate out-
crossing) by exposing them to ethyl methane-
sulfonate (EMS) to infuse novel genetic variation
in each population. The five replicate populations
were then passaged under three different para-
site treatments (table S1): (i) control (no exposure
to S. marcescens), (ii) evolution (repeated expo-
sure to a fixed, nonevolving strain of S. marcescens),
and (iii) coevolution. The coevolution treatment in-
volved repeated exposure (30 host generations) to
a potentially coevolving population of S. marcescens,
which was under selection for increased infectiv-
ity. S. marcescens Sm2170 served as the ancestral
strain in the coevolution treatment, as well as the
fixed strain in the evolution treatment.
The results were consistent with the Red
Queen hypothesis. In the coevolution treatment,
all of the obligately selfing populations became
extinct within 20 generations (fig. S1). However,
S. marcescens
Ancestor
Non-coevolving
Coevolving
Obligately Selfing C. elegans
b
a
Ancestral Populations
Generation 10
"Coevolution" Populations
Wildtype (Mixed Mating) C. elegans
g
Ancestral Populations
Generation 30
"Coevolution" Populations
Obligately Outcrossing C. elegans
m
Ancestral Populations
n
Generation 30
"Coevolution" Populations
REPORTS
none of the obligately selfing populations went
extinct in either the evolution treatment or in the
control treatment. In addition, all of the obligately
outcrossing and wild-type populations persisted
throughout the experiment in all three treatment
types (fig. S1). Thus, extinction was only ob-
served in obligately selfing hosts when confronted
with coevolving pathogens.
We also found that the presence of coevolving
S. marcescens selected for and maintained high
levels of outcrossing in wild-type C. elegans pop-
ulations (Fig. 1). Over the first eight generations
of the experiment, outcrossing rates increased
from 20% to more than 70% in both the evo-
lution and coevolution treatments (Fig. 1) (F2,11=
8.26; P = 0.006). However, the wild-type popu-
lations consistently exposed to a fixed population
of S. marcescens (evolution treatment) exhibited
a steady decline in outcrossing rates after this ini-
tial increase, eventually returning to control levels
of outcrossing (Fig. 1), as previously observed (5).
In contrast, populations in the coevolution treat-
ment consistently maintained high levels of out-
crossing throughout the experiment, relative to
the control treatment (Fig. 1) (F1,12 = 209.5; P<
0.0001). Coevolution with S. marcescens, there-
fore, favored the evolution and long-term main-
tenance of higher rates of outcrossing.
As also predicted by the Red Queen hypoth-
esis, outcrossing hosts adapted to changes in the
pathogen population, whereas selfing apparently
prevented an adaptive counter-response. The an-
cestral strain of the obligately selfing hosts suffered
higher mortality rates when exposed to bacteria
from the coevolution treatment than when ex-
posed to either the ancestral bacteria (Fig. 2A)
(c> a: F1,71 = 21.2; P<0.0001) or to the nonco-
evolving control bacteria (Fig. 2A) (c>b: F1,71 =
31.9; P<0.0001). Therefore, the bacteria in the
coevolution treatment evolved greater infectivity
in response to selection. Further, the obligately
selfing hosts did not adapt to the evolution of
increased infectivity in the bacteria, because
the bacteria from the coevolution treatment in-
duced greater levels of mortality against the worms
after 10 generations of coevolution than against
the ancestral hosts (Fig. 2A) (f>c: F1,71 = 69.2;
P<0.0001). Finally, an increase in mortality
by more than a factor of 3 was observed in the
obligately selfing hosts in only 10 generations
(Fig. 2A) (f> a: F1,71 = 173.7; P < 0.0001),
which could explain why these hosts were driven
to extinction.
The pathogens that coevolved with the wild-
type and obligate outcrossing populations also
evolved greater infectivity (Fig. 2, B and C) (i > h:
F1,104 69.5; P<0.0001; i >g: F1,104 = 32.9; P<
0.0001; on: F1,60 = 141.1; P<0.0001; o > m:
F1,60 50.9; P<0.0001). However, the wild-type
and obligately outcrossing populations adapted
to the changes in their respective coevolving path-
ogen populations. Specifically, both the wild-type
and obligately outcrossing populations exhibited
lower mortality rates against the pathogens with
which they were currently evolving than did their
www.sciencemag.org SCIENCE VOL 333 8 JULY 2011
217
Downloaded from www.sciencemag.org on March 19, 2012
REPORTS
218
ancestors (Fig. 2, B and C) (i >l: F1,104=27.9; P<
0.0001; o>r: F1,60 = 166.2; P<0.0001), thus
indicating reciprocal coevolution in the outcross-
ing host populations. Whereas the obligate selfing
populations in the coevolution treatment became
more infected over time (Fig. 2A), the wild-type
populations maintained the same level of infec-
tivity over the course of the experiment (Fig. 2B)
(g=l: F1,104 0.35; P=0.554), while the obligate
outcrossing populations were significantly less
infected at the end of the experiment relative to
the beginning (Fig. 2C) (m>r: F1,60 = 33.1; P<
0.0001). Coupled with the maintenance of high
outcrossing rates in the coevolving wild-type
populations (Fig. 1), these results demonstrate the
ability of antagonistic coevolution to continually
generate novel environmental conditions under
which outcrossing is favored and populations per-
sist when interacting with a virulent pathogen.
A recent host/pathogen coevolution study in
C. elegans further supports the conclusion that
low levels of outcrossing impede the rate of
adaptive evolution. The C. elegans hosts in this
previous study appear to have primarily repro-
duced via self-fertilization and did not evolve
significantly greater resistance to a coevolving
pathogen over 48 generations of selection (27).
Contrary to our study, however, greater out-
crossing rates did not evolve in these mixed-
mating populations in response to the pathogen.
It may be that higher levels of genetic variation
and/or a greater level of pathogen virulence in
our study account for the difference in outcomes.
In summary, we found that obligately selfing
lineages were driven to extinction when con-
fronted with a coevolving parasite. These results
are consistent with the macroevolutionary aspects
of the Red Queen hypothesis, as originally formu-
lated by Van Valen (28). We also found that the
presence of a coevolving pathogen selected for and
maintained high levels of outcrossing in mixed-
mating populations, whereas elevated levels of
outcrossing were not maintained in populations
where the pathogen was not coevolving. These
results are consistent with the microevolutionary
predictions of the Red Queen. Taken together, the
results demonstrate that sex can facilitate adap-
tation to novel environments, but the long-term
maintenance of sex requires that the novelty does
not wear off.
References and Notes
1. G. C. Williams, Sex and Evolution (Princeton University
Press, Princeton, NJ, 1975).
2. J. Maynard Smith, The Evolution of Sex (Cambridge
University Press, Cambridge, UK, 1978).
3. G. Bell, The Masterpiece of Nature: The Evolution and
Genetics of Sexuality (University of California Press,
Berkeley, CA, 1982).
4. G. L. Stebbins, Am. Nat. 91, 337 (1957).
5. L. T. Morran, M. D. Parmenter, P. C. Phillips, Nature 462,
350 (2009).
ature blood cell lineages are generated
from a network of hierarchically dis-
tinct progenitors that arise from self-
renewing hematopoietic stem cells (HSCs). The
extensive regenerative potential of HSCs makes
them attractive targets for cellular and genetic
6. H. J. Muller, Am. Nat. 66, 118 (1932).
7. R. A. Fisher, The Genetical Theory of Natural Selection
(Clarendon Press, Oxford, 1930).
8. R. Lande, D. W. Schemske, Evolution 39, 24 (1985).
9. D. Charlesworth, B. Charlesworth, Annu. Rev. Ecol. Syst.
18, 237 (1987).
10. A. F. Agrawal, C. M. Lively, Evolution 55, 869 (2001).
11. J. Jaenike, Evol. Theory 3, 191 (1978).
12. W. D. Hamilton, Oikos 35, 282 (1980).
13. W. Hamilton, R. Axelrod, R. Tanese, Proc
Sci. U.S.A. 87, 3566 (1990).
14. C. M. Lively, Nature 328, 519 (1987).
Isolation of Single Human Hematopoietic
Stem Cells Capable of Long-Term
Multilineage Engraftment
Faiyaz Notta,¹,²* Sergei Doulatov, ¹,2* Elisa Laurenti, ¹,² Armando Poeppl,¹
Igor Jurisica,3,4 John E. Dick¹, ²+
Acad.
Lifelong blood cell production is dependent on rare hematopoietic stem cells (HSCs) to
perpetually replenish mature cells via a series of lineage-restricted intermediates. Investigating
the molecular state of HSCs is contingent on the ability to purify HSCs away from transiently
engrafting cells. We demonstrated that human HSCs remain infrequent, using current purification
strategies based on Thy1 (CD90) expression. By tracking the expression of several adhesion
molecules in HSC-enriched subsets, we revealed CD49f as a specific HSC marker. Single CD49f+
cells were highly efficient in generating long-term multilineage grafts, and the loss of CD49f
expression identified transiently engrafting multipotent progenitors (MPPs). The demarcation of
human HSCs and MPPS will enable the investigation of the molecular determinants of HSCs,
with a goal of developing stem cell-based therapeutics.
therapies. The molecular regulation of specific
HSC properties such as long-term self-renewal is
beginning to be elucidated for murine HSCs (1).
However the biology of human HSCs remains
poorly understood because of their rarity and the
lack of methods to segregate HSCs from multip-
8 JULY 2011 VOL 333
15. K. C. King, L. F. Delph, J. Jokela, C. M. Lively, Curr. Biol.
19, 1438 (2009).
16. C. M. Lively, C. Craddock, R. C. Vrijenhoek, Nature 344,
864 (1990).
17. E. Decaestecker et al., Nature 450, 870 (2007).
18. B. Koskella, C. M. Lively, Evolution 63, 2213 (2009).
19. J. Jokela, M. F. Dybdahl, C. M. Lively, Am. Nat. 174
(suppl. 1), S43 (2009).
20. S. Paterson et al., Nature 464, 275 (2010).
21. S. Brenner, Genetics 77, 71 (1974).
22. H. Teotónio, D. Manoel, P. C. Phillips, Evolution 60, 1300
(2006).
23. L. M. Miller, J. D. Plenefisch, L. P. Casson, B. J. Meyer,
Cell 55, 167 (1988).
24. T. Schedl, J. Kimble, Genetics 119, 43 (1988).
25. C. L. Kurz et al., EMBO J. 22, 1451 (2003).
26. G. V. Mallo et al., Curr. Biol. 12, 1209 (2002).
27. R. D. Schulte, C. Makus, B. Hasert, N. K. Michiels,
H. Schulenburg, Proc. Natl. Acad. Sci. U.S.A. 107, 7359
(2010).
28. L. Van Valen, Evol. Theory 1, 1 (1973).
Acknowledgments: We thank H. Hundley and R. Matteson
for logistical assistance. We also thank F. Bashey,
L. Delph, P. Phillips, M. Parmenter, the Lively and Hall
laboratories, and two reviewers for helpful comments
and discussion, as well as the Wissenschaftskolleg zu
Berlin for a fellowship to C.M.L. during the preparation
of the manuscript. Funding was provided by the NSF
(DEB-0640639 to C.M.L) and the NIH (1F32GM096482-01
to L.T.M). Nematode strains were provided by the
Caenorhabditis Genetics Center, which is funded by the
NIH National Center for Research Resources (NCRR). Data
deposited at Dryad, 10.5061/dryad.c0q0h.
Supporting Online Material
www.sciencemag.org/cgi/content/full/333/6039/216/DC1
Materials and Methods
Fig. S1
Table S1
References 29 to 31
31 March 2011; accepted 24 May 2011
10.1126/science.1206360
otent progenitors (MPPs) to obtain pure popula-
tions for biological and molecular analysis.
The bulk of HSCs are CD34+, as evidenced
by human transplantation and xenograft re-
population assays; however, most CD34+ cells
are lineage-restricted progenitors and HSCs re-
main rare. HSCs can be enriched further on the
basis of CD45RA (2), Thy1 (3-5), and CD38
(6, 7) expression. Loss of Thyl expression in the
CD34 CD38 CD45RA compartment of lineage-
depleted cord blood (CB) was recently proposed
to be sufficient to separate HSCs from MPPS
(5). However, more than a third of Thy1 primary
recipients gave rise to engraftment in secondary
animals, raising uncertainty about whether Thyl
can absolutely segregate HSCs from MPPs. To
¹Division of Stem Cell and Developmental Biology, Campbell
Family Institute for Cancer Research/Ontario Cancer Institute,
Toronto, Ontario, Canada. ²Department of Molecular Genetics,
University of Toronto, Toronto, Ontario, Canada. ³Ontario
Cancer Institute and Campbell Family Institute for Cancer
Research, Toronto, Ontario, Canada. Departments of Com-
puter Science and Medical Biophysics, University of Toronto,
Toronto, Ontario, Canada.
*These authors contributed equally to this work.
To whom correspondence should be addressed. Toronto
Medical Discovery Tower, Room 8-301, 101 College Street,
Toronto, Canada M5G 1L7. E-mail: jdick@uhnres.utoronto.ca
SCIENCE www.sciencemag.org
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax