What is the dose (in gray and rem) that the tumor receives? What is the dose (in milligray and rem) that the rest of the tissues receives?
Q: Electrostatics : A solid conducting sphere of radius a carries a net positive charge 3Q. A conductin...
A: Basic Details The electric field strength depends on the coulombs constant, the net charge in the re...
Q: Please explain this problem please.
A: Given information: Here, f is the frequency of the antenna.
Q: Make sure to consider all parts of the problem with detail and accuracy. Please show your work and e...
A:
Q: 13. a) Natural potassium contains 40K , which has a half-life of 1.277×109years. What mass of 40K in...
A: a) The number of radioactive nuclei is,
Q: A radiation worker stands 50 cm away from a radioactive source of 25 mCi 57Co. Calculate the exposur...
A: Given the activity of the radioactive source is equal to 25 mCi . The half- life of the source is T1...
Q: Electric lines of forces are produced by three point charge arranged vertically as reveals in Fig. T...
A:
Q: What mass of thallium-201 is needed to have an activity of 10 mCi? How long will it take for the act...
A:
Q: When ultraviolet light with a wavelength of 400.0 nm falls on a certain metal surface, the maximum ...
A:
Q: 9- Please I want answer for this question by typing it. Thanks
A:
Q: Find the electric field distance r at point due a point charge Q which is filled with dielectriccon...
A:
Q: The radioactive nuclide Plutonium-199 has a half-life of 43.0 min. A sample isprepared that has an i...
A:
Q: Make sure to consider all parts of the problem with detail and accuracy. Please show your work and e...
A: As per guidelines, the first three sub-parts have been answered.
Q: Typical 10-fold intensity reduction values for X-ray radiation with energy 1 MeV are 30 cm for bone ...
A: Energy of the x ray radiation is equal to 1 MeV. Thickness of the bone tissue is 30 cm and thickness...
Q: Photoelectrons from a material with a binding energy of 2.71 eV are ejected by 420-nm photons. Once ...
A:
Q: Explain diffraction grating by Young's double slit experiment
A:
Q: Figure 29-33 shows four identical currents i and five Amperian paths (a through e) encircling them. ...
A:
Q: 10///
A:
Q: Particle A of charge 2.85 10-4 C is at the origin, particle B of charge -5.64 10-4 C is at (4.00 m, ...
A:
Q: The Particle-Wave Duality Reviewed• Explain the concept of particle-wave duality, and its scope.
A: In 1924 Louis de Broglie suggested that all matter has a wave like nature. Hence all particles wit...
Q: A particle has y=18,399. Calculate c-v in m/s. (I would have asked for 1- v/c, making the answer dim...
A:
Q: Describe what the sky would look like at the North Pole at night. How would the sky change over a 2...
A: The sky looks different from the different parts of the earth. Its depend on what is your position o...
Q: Calculate the radiation dose in rem per year (365 days) for the lungs of a weapons plant employee wh...
A:
Q: Consider an astronaut traveling to another star at a relativistic velocity. Construct a problem in w...
A: Assume the speed of the spaceship to be 0.9c and the distance to the star to be 5 light years and ma...
Q: A particle is projected as shown in figure.a) Determine the range of values of the initialspeed to b...
A: The figure for the given problem,
Q: A 60-kg person accidentally ingests a small source of alpha particles (RBE=15). The activity of the ...
A:
Q: Find the moment of inertia of a solid cylinder of mass m, length l and radius R about the following ...
A:
Q: 1.A silver wire 2.6 mm in diameter transfers a charge of 420 C in 80 minutes. Silver contains 5.8 x ...
A: (a) Current in the wire
Q: 2/
A:
Q: A 15.0 kg block is attached to a very light horizontal spring of force constant 575 N/m and is resti...
A:
Q: 10- Please I want answers of sub-part (b) by typing it. Many Thanks
A:
Q: 2 part question.
A:
Q: The charges are enclosed with in a surface at which neither the charge distribution nor the potentia...
A: Estimating the electric field: Let Charges enclosed by the conducting surface are Q1 and Q2 for whi...
Q: Quantum Numbers and Rules• Define quantum number.• Calculate angle of angular momentum vector with a...
A: The quantum number represents the energy levels available for atoms and molecules. The state of an e...
Q: A particle has γ=18,399. a)Calculate c-v in m/s. (I would have asked for 1 - v/c, making the answer ...
A:
Q: Please I want answer of this Electrical Principles question. Thanks
A: Given information: Here, Req1 and Req2 are the required resistances.
Q: Two cylinders are connected by a string that is wrapped around each cylinder. If the cylinder is fix...
A: a) Torque about the second mass, Now, net force for the second mass,
Q: The probabi tiy particle is in the nd stete Cn-1) that the gro The probobulity thal the partick is i...
A:
Q: Q2. A static electric device works at 240 V uses a current of 0.64 A connected to a 20 V,70 W comput...
A: The static device is converting the high voltage into a low voltage. Therefore it can be a step-down...
Q: We have a copper conductor, length l = 1m and circular section of diameter d = 4mm a) Calculate the ...
A: Part (a) Basic Details The resistance of the conductor depends on the resistivity, the length of the...
Q: 2. Sketch the f v.s. Uind graph for given data table. Determine the value of po and calculate the er...
A: The graph for the given table is as follows:
Q: A rectangular waveguide which has a cross section of a = 6 cm and b = 3 cm is filled with a magnetic...
A: Basic Details The cut off frequency of the wave guide depends on the cross section of the rectangula...
Q: 38. Gravitational field a. Find a potential function for the gravitational field xi + yj + zk F = -G...
A: (a) Let’s write force for any potential V,
Q: A -4.00 nC point charge is at the origin, and a second -5.00 nC point charge is on the x-axis at x =...
A:
Q: Typical 10-fold intensity reduction values for X-ray radiation with energy 1 MeV are 30 cm for bone ...
A:
Q: 5. What is the exposure rate at 30 cm from a vial containing 20 mCi (740 MBq) of 131I?
A:
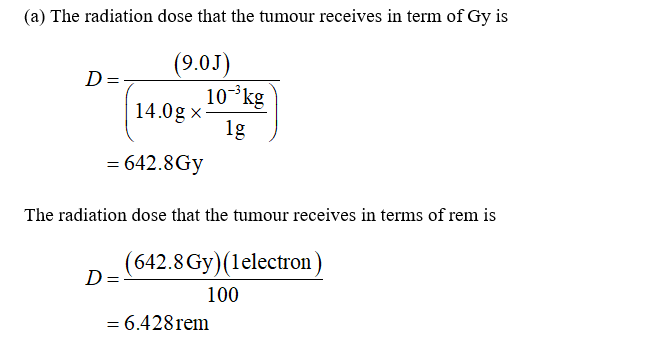
Q: A 14-gram ovarian tumor is treated using a sodium phosphate solution in which the phosphorus atoms a...
A: (i) Absorbed dose by the tumor
Q: A series circuit contains the following components: R=160ohms, L=200mH, C=20uF, and a generator with...
A:
Q: Question is on the picture. It's IB Physics SL, so I wasn't sure what category to put it in.
A:
A 14-gram ovarian tumor is treated using a sodium phosphate solution in which the phosphorus atoms are the radioactive Phosphorus-32 isotope with a half-life of 14. 3 days and which decays via beta emission (RBE=1) with an energy of 1.71MeV. Half of the sodium phosphate solution is absorbed by the tumor and deposits 9.0 J of energy into it. The other half of the solution is dispersed throughout the patient’s tissues, also depositing 9.0 J of energy into the 50.0 kg of body tissues.
What is the dose (in gray and rem) that the tumor receives?
What is the dose (in milligray and rem) that the rest of the tissues receives?
Step by step
Solved in 2 steps with 2 images
