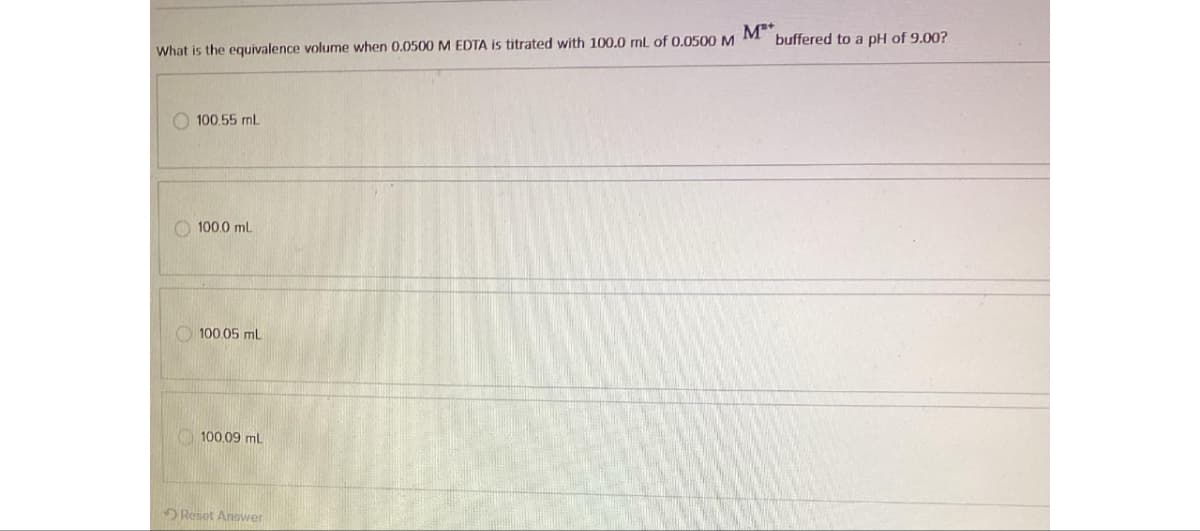
What is the equivalence volume when 0.0500 M EDTA is titrated with 100.0 mL of 0.0500 M M 100.55 mL 100.0 mL 100.05 mL 100.09 mL buffered to a pH of 9.00?
Q: 1. What is the concentration of the OH- ion in 0.03 M HCl? 2. What molarity of butanoic acid (pka) =…
A: Here, HCl is a strong acid and butanoic acid is a weak acid.
Q: write a lab report with reference on salicylic acid titration (1500 words)
A: The aim of this question is to show the salicylic acid titration. It is an bulky drug which contain…
Q: QUESTION 1 Calculate the molar mass of carbonic acid (H2CO3). There may be slight differences…
A: Calculation of the molar mass of carbonic acid is given below.
Q: 9 Complete the following reactions by briefly explaining in each case the type of reaction taking…
A: The given reaction involves H2 and Pd/C, which is a common setup for a hydrogenation reaction.…
Q: Which of the following reactions best describes the dissolution of gaseous hydrogen chloride (HCI)…
A: we have to find the reaction that best describes the dissolution of gaseous hydrogen chloride (HCl)…
Q: combination
A: Glycosphingolipids are a type of complex sphingolipids that are composed of a ceramide and one Or…
Q: Provide the major organic product(s) of the reaction below. H3C CH3 H3C H 1. LiAlH4 2. H, H₂O
A:
Q: Given is the following redox half equation. Name the Mistake (3), give an advice to avoid those…
A: The objective of the question is to identify the mistakes in the given redox half equation and…
Q: e. CH3 CH3 C=C= CH3 -CH3 Cl₂ -CH3 CC14 CH3 h C11H17CI
A: The objective of this question is to show the Chlorination reaction in presence of solvent carbon…
Q: Fure water dissociates sightly and forms a small concentration of H' and CH ors. The value of Kat…
A: Autoionisation of water is the reversible reaction in which water molecules are dissociated…
Q: Construct a figure of Lineweaver-Burk plot. Determine the K, and Vmx of the enzyme- substrate…
A: The 1/S and 1/V values are tabulated below :Substrate concentration(mM)Velocity(mM/min)1/S…
Q: 15. What type of nucleophilic aromatic substitution is the reaction of m-dinitrophenol with NaOH…
A: The objective of the question is to predict the type of nucleophilic aromatic substitution for the…
Q: thank you very much, but you did not show the mechanism for product A and B for first question, you…
A: The Friedel-Craft Acylation is the reaction in which an acyl group is introduced into an aromatic…
Q: Robinson Annulation - Practice Compound 1 is a key intermediate in the synthesis of female hormone…
A: Since you have posted a question with multiple sub parts, we will provide the solution only to the…
Q: A chemistry student needs 15.0 g of ethanolamine for an experiment. By consulting the CRC Handbook…
A: According to the question,The student needs the mass of ethanolamine = 15.0 g.The density of the…
Q: (b) Propose a possible catalytic cycle to account for the following transformation and the oxidation…
A: Heck Reaction is the coupling reaction of C-C in presence of palladium catalyst between aryl halides…
Q: Nitrogen monoxide reacts with hydrogen at 500 °C as in the equation below. 2NO(g) + 2H₂(g) → N₂(g) +…
A: The given reaction is:Let us find the order of reaction for each reactant.To find the order of…
Q: In a coffee cup calorimeter, 32.0 mL of 0.71 M nitric acid (HNO3) and 32.0 mL of 0.71 M potassium…
A: The objective of this question is to calculate the ∆Hrxn by using given data. The calorimetry method…
Q: How is it used in 21. Injection of ethane, propane, butane, pentane, hexane, hep- tane, and octane…
A: The aim of this question is to show the ethane, propane, butane, pentane, hexane, heptane, and…
Q: Consider the molecules H₂O, H₂S, and CO₂. Match the following dipole moments to those three…
A:
Q: Draw the complete structural formula and condensed molecular formula for propene
A: In the condensed formula, we do not show any Bond. We explicitly draw the atoms of the side chain in…
Q: Show the product for the following reaction:
A: The objective of this reaction which involved hydroxy formation in given starting material. It…
Q: d. CH3 CH3 + NBS A peroxide
A: The question is based on organic reactions.We need to identify the product and explain its…
Q: Protein sources that provide all the amino acids that cannot be manufactured in the body are…
A: Proteins are fundamental building blocks of life, playing a pivotal role in the structure and…
Q: What is the name of the following compound? CH3 Br A) m-bromomethylbenzene B) m-bromotoluene C)…
A: For aromatic compounds, the name of the parent ring has to be identified.The numbering starts from…
Q: 1. The co-ordination number of metal crystallizing in a hexagonal close packed structure is (a) 12…
A: The question addresses the coordination number of a metal crystallizing in a hexagonal close-packed…
Q: Write the organic product(s) of the following SN1 reactions. Indicate cis or trans, or R or S or…
A:
Q: Question 6.a of 12 Ethers are a class of organic compounds that contain an oxygen atom connected to…
A: The above question is based on IUPAC nomenclature of organic compounds. Let's solve the above…
Q: Consider the following two possibilities for electron tran- sitions in a hydrogen atom, pictured…
A:
Q: Write the net ionic equation to show the formation of a solid (insoluble ionic compound) when the…
A: The objective of this question is to explain the net Ionic equation to show the formation of a solid…
Q: For the following optimisation step, the affinity and potency of compound will be affected mainly…
A: In summary, both enthalpy and entropy can influence the affinity and potency of a drug or ligand.…
Q: Consider the following ions: NO₂, CO32, and PO,³. For each of these, you should use a Lewis…
A: Resonance structures are a set of Lewis structures, which collectively describe the electron bonding…
Q: If I mix 34.0 mL of 2.00M silver nitrate with 66.0 mL of 1.18 M sodium chloride, what is the…
A: In given question reaction is taking place between silver nitrate and sodium chloride. Which a…
Q: Calculate the pH change when 50 mL 0.100 M HCI is added to 50 mL 0.100 M NH4OH (5.27 \times 10^-6)
A: Volume of HCl and NH4OH = 50.0 mLMolarity of HCl and NH4OH = 0.100 M
Q: Make a buffer using 16g NaOH (MW = 40g/mol) with 300ml H3PO4 1M and then dilution of the compound to…
A: The objective of this question is to calculate the pH of a buffer solution made by mixing NaOH and…
Q: (c) An octahedral cluster molecule E6 in Oh point symmetry can be formed without a central atom and…
A: The objective of this question is to determine the three irreducible representations for the…
Q: q(t) h(t) h .g. (t) qo= R! To establish the non-sedentary and sedentary mass balance for the liquid…
A:
Q: 1. (A) HO OH (B) NHẠNH, KOH, heat (C)
A: In question 1, the 1st example is the ketal formation reaction, the 2nd example is the imine…
Q: 3.8801 g of zinc metal was dissolved in dilute sulphuric acid and then made up to 381 cm3 with…
A: The objective of the question is to find the percentage mass in volume composition of zinc sulphate…
Q: CO(g) + Cl₂(g) →→CoCl₂2(g) Using the standard thermodynamic data in the tables linked above,…
A: We calculate the standard free energy change for the reaction by subtracting the summation of the…
Q: The synthesis of compound M from compound J is shown below: C6H100 K H + NaOH (excess) reaction a…
A: In structure y The carbon which is present adjecent to the carbonyl carbon is called alpha carbon…
Q: 14 Question 10 What is the Ka of an acid if a 0.057M solution of the acid has pH = 4.45? X 10^…
A: A balanced chemical equation for the partial ionization of the acid is HA(aq) + H2O(l) H3O+(aq) +…
Q: reduction of reducible Representation to irreducible representation of C4 V point group, Raman, IR…
A: In group theory, the reducible representation (Γ) of a molecule can be decomposed into irreducible…
Q: Which of the following functional groups is the most stable to metabolism? a. O b. O c. O d. HO HN…
A: For the stability of functional group towards metabolism we need to check the stability using the…
Q: solve by drawing out the molecules and showing the bonds relation to this question 2. How much…
A:
Q: Given 0.01 M solutions of each of the following acids, which solution would have the lowest pH? O…
A: The objective of the question is to determine the solution with the lowest pH.
Q: Name the following compound (hint: expand the formula before you answer there are total four CH2)…
A:
Q: The derivation of the movement of chemical assumed that chemical moved as easily into the cell as…
A: The objective of this question is to explain the different ways in which chemicals can move between…
Q: Figure 7 of Conrad et al. (1999) shows the variation in C-isotopic composition of CO2 as a function…
A: The purpose of this this question is to measurements was to assess the environmental effects of jet…
Q: Provide the missing structures B, C, and D and the reagents A and E. No mechanisms required. (3…
A: The first step of this reaction is the, formation of Pyridine-N -oxide , when Pyridine react with…
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

- answer the following: Let us assume that there is a 0.020 M EDTA titrant solution. Calculate the mass of pure dry CaCO3 standard to use such that the volume of titrant needed to reach the endpoint will be about 35mL if one mole of calcium carbonate reacts with one mole EDTA. Given choices: 0.050 g 0.060 g 0.070 g 0.080 gThe data from my observations is given below: Concentration of Standardized NaOH - 1.00 mol/L Mass concentration of acetic acid - 2.40 g/L Trial 1 Trial 2 Measured pH of the acetic acid solution 3.07 3.05 Mass of acetic acid solution taken for titration 20.0 g 20.0 g Initial buret reading of NaOH titrant 0.00 mL 0.00 mL Final buret reading of NaOH titrant 0.82 mL 0.79 mL Net volume of NaOH 0.82 mL 0.79 mL Millimoles of NaOH to end point of titration 0.82 mmol 0.79 mmol Millimoles of acetic acid in sample 0.82 mmol 0.79 mmol Molar concentration of acetic acid solution 0.043 mol/L 0.041 mol/L Calculated molar mass of acetic acid g/mol g/mol Average molar concentration of acetic acid for the samples titrated: 0.042 mol/L Average pH: 3.06 Question: Calculate the corresponding [H3O+], compute the corresponding concentration of A- and HA, and calculate the dissociation constant Ka Thank you!A RbOH solution is titrated four (4) times against potassium hydrogen phthalate (KHP; FW=204.224) samples to the Phenolphthalein endpoint. Using the data below, determine the concentration of the RbOH solution? g of KHP Volume of Base Required 0.5373 g 42.49 mL 0.5856 g 43.88 mL 0.5790 g 48.56 mL 0.5856 g 44.60 mL (Report your answer as "mean +/- std dev") M What is the percent relative standard deviation? % What is the 99% Confidence Interval for the concentration of the solution (population mean)?
- You want to measure the concentration of carbonate (CO32-) in a mildly basic solution by using an EDTA back titration. CaCO3 has a Ksp of 5x10-9. You add 50.00 mL of 0.3484 M CaCl2 to 500.0ml of sample and filter the solution to remove the precipitate. You then take 250.0 mL of the filtered solution and titrate with 0.1786 M EDTA. You require 23.72 mL to reach the endpoint. What is the concentration of carbonate in the original sample?Answer if true or false , if false, put the correct answer 1. There is 11.34 mmol in 0.2011 of 0.5604 M HgCl2 2. The x axis of sigmoidal titration curve is p function of the analyte or titrant 3.The concentration of the secondary standard is relative to a primary standard 4. There is 110 mmol in 79.8 mL of 0.1379 M NH4VO3 (116.98 g/mol)At equivalence point in the titration of M2+ with EDTA, A. [M2+] = [Y] B. [M2+] = [Y4-] C. No answer D. [M2+] = [MY2-] E. [MY2] = [Y]
- Titration of 50.00 mL of 0.04715 M Na2C2O4 required 39.25 mL of a potassium permanganate solution.MnO4- + 5H2C2O4 + 6H+ → 2Mn2+ + 10CO2(g) + 8H2OCalculate the molar concentration of the KMnO4 solution.Titration of 50.00 mL of 0.04715 M Na2CO3 required 39.25 mL of potassium permanganate solution. 2MnO4- + 5H2C2O4+ + 6H+ 2Mn2+ + 10CO2 (g) + 8H2O Calculate the molar concentration of KMnO4 solution (6)The end point of a titration was reached after 22.2 mL22.2 mL of 0.050 M0.050 M disodium EDTA titrant was dispensed into a solution containing the zinc ion. Calculate the moles of disodium EDTA used. moles of disodium EDTA: = mol
- 2,5 ml volume has taken from an “hypothetic” solution which includes (3+) Sb and (3+) Fe and at the titration with 0.1004 N KMnO4, the wasted amount has found as 16,4 mL. The other 2,5 mL that has taken, has reduced with Zn after that, this 2,5 mL solution has titrates with the same KMnO4 solution solution and the wasted amount is 26,5mL. With these datas find the %concentrations of the ions at the solution.Calculate the pH at 0.0, 10.0, 25.0, 50.0, and 60.0 mL of titrant in the titration of 50.0 mL of 0.100 M NH3 with 0.100 M HCl. Pls answer thank youAt 40.0 mL 0.0050 M Sr2 + pH 10, 0.0100 M EDTA is titrated. Calculate the pSr for the equivalence point. Kfor (SrY) = 4.3x108, α4 = 0.35

