What is the formula weight of the compound? (Mg = 24.31 amu, Cl = 35.45 amu, O = 16 amu, H = 1.01 amu) * MgCl2 · 6H20 561.2 466.8 O 123.33 203.3 186,353 When iron pyrite (FeS2) is heated in air, the process known as "roasting" forms sulfur dioxide and iron(III) oxide. When the equation for this process is completed and balanced, using the smallest whole number coefficients, what is the coefficient for "O2"? * Fes2 + O2 SO2 + Fe2O3 7 8 11 O 4
What is the formula weight of the compound? (Mg = 24.31 amu, Cl = 35.45 amu, O = 16 amu, H = 1.01 amu) * MgCl2 · 6H20 561.2 466.8 O 123.33 203.3 186,353 When iron pyrite (FeS2) is heated in air, the process known as "roasting" forms sulfur dioxide and iron(III) oxide. When the equation for this process is completed and balanced, using the smallest whole number coefficients, what is the coefficient for "O2"? * Fes2 + O2 SO2 + Fe2O3 7 8 11 O 4
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 7ALQ: You may have noticed that when water boils, you can see bubbles that rise to the surface of the...
Related questions
Question
100%
Hello please help me with this... this is just short. no need for solution with the number with solving
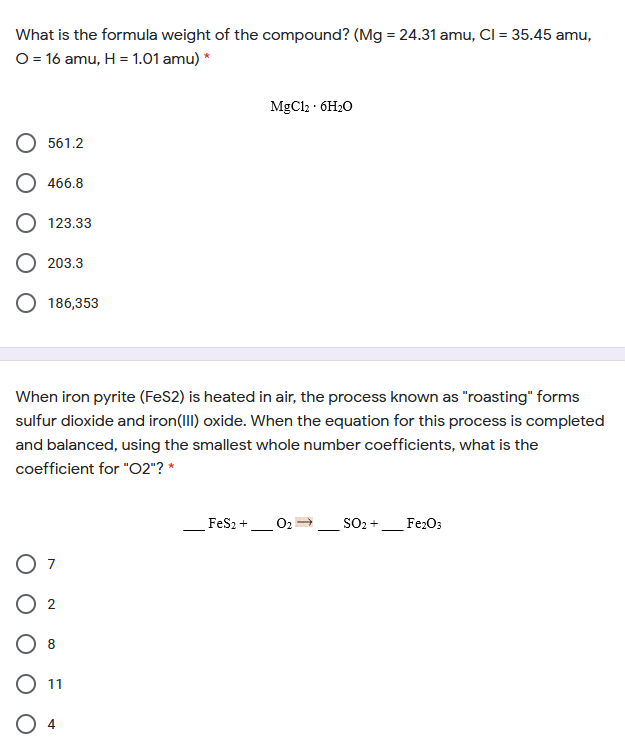
Transcribed Image Text:What is the formula weight of the compound? (Mg = 24.31 amu, Cl = 35.45 amu,
O = 16 amu, H = 1.01 amu) *
MgCl2 · 6H20
561.2
466.8
O 123.33
203.3
186,353
When iron pyrite (FeS2) is heated in air, the process known as "roasting" forms
sulfur dioxide and iron(III) oxide. When the equation for this process is completed
and balanced, using the smallest whole number coefficients, what is the
coefficient for "O2"? *
Fes2 +
O2
SO2 +
Fe2O3
7
8
11
O 4

Transcribed Image Text:Identify the INCORRECT statement.
Tap water: a compound
Helium in a balloon: an element
Mercury in a barometer: an element
Paint: a mixture
9 This is a required question
Which answer includes all the following that are chemical changes and not
physical changes? *
I. freezing of water
II. rusting of iron
III. dropping a piece of iron into hydrochloric acid (H2 is produced)
IV. burning a piece of wood
V. emission of light by a kerosene oil lamp
O II, II, V
O Il and V
O 1, II, II, IV and V
O IIl and IV
II, III, IV and V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning