What is the functional group of propanoic acid? How do we know if it's soluble in water or insluble in water using the flowchart that is attached. which test should be performed using the flowchart. Is propanoic acid a ketone, aldehyde, ester or alcohol and how did you know?what would be the most efficient series of solubility and functional group tests that would identify the functional group? Also what could be the sources of error in this experiment? What could occur that would give false positives, false negatives, or incorrect interpretations
What is the functional group of propanoic acid? How do we know if it's soluble in water or insluble in water using the flowchart that is attached. which test should be performed using the flowchart. Is propanoic acid a ketone, aldehyde, ester or alcohol and how did you know?what would be the most efficient series of solubility and functional group tests that would identify the functional group? Also what could be the sources of error in this experiment? What could occur that would give false positives, false negatives, or incorrect interpretations
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter16: Solutions
Section: Chapter Questions
Problem 100E: Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a...
Related questions
Question
What is the
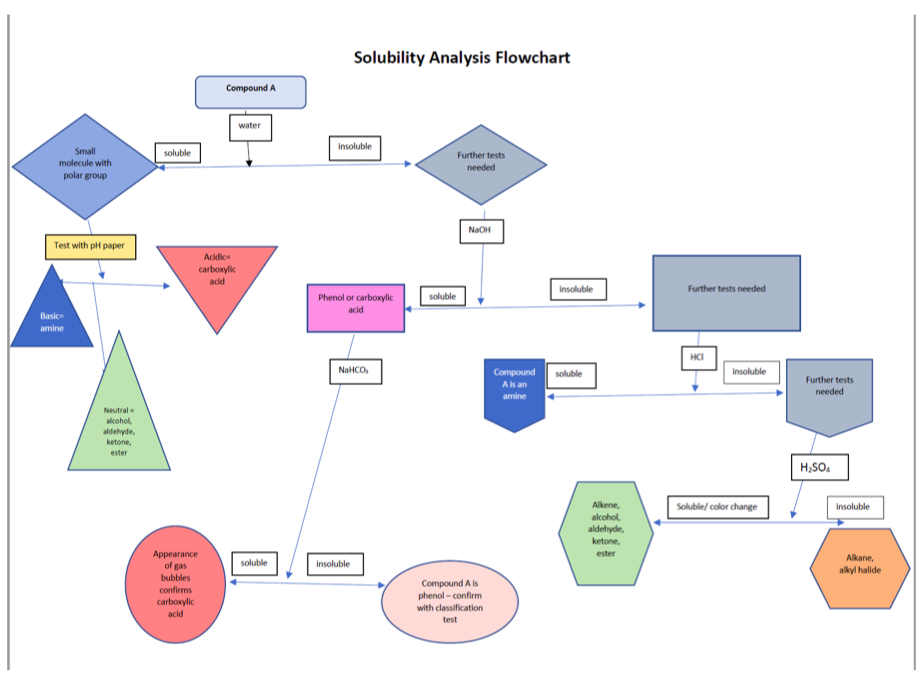
Transcribed Image Text:Solubility Analysis Flowchart
Compound A
water
Insoluble
Small
soluble
Further tests
needed
molecule with
polar group
NaOH
Test with pH paper
Acidic
carboxylic
acid
Insoluble
Further tests needed
Phenol or carboxylic
soluble
acid
Basic
amine
HCI
Compound soluble
NaHCO,
insoluble
Further tests
A is an
needed
amine
Neutral
alcohol,
aldehyde,
ketone,
ester
H;SO.
Soluble/ color change
Alkene,
alcohol,
aldehyde,
ketone,
Insoluble
Аppearance
of gas
ester
Alkane,
soluble
Insoluble
alkyl halide
bubbles
Compound A is
confirms
carboxylic
acid
phenol - confirm
with classification
test
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning