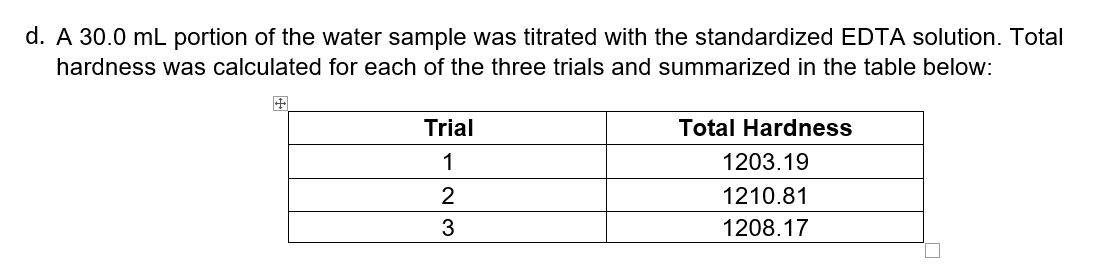
d. A 30.0 mL portion of the water sample was titrated with the standardized EDTA solution. Total hardness was calculated for each of the three trials and summarized in the table below: Trial Total Hardness 1 1203.19 2 1210.81 1208.17
PS. Further values required for the solvings are give in the various situations below. (ANSWER)
Situation: A community in a mountainous area of Bohol uses water collected from a nearby natural spring. A sample was submitted to a laboratory for the analysis of its total hardness.
Required: SHOW YOUR COMPLETE CALCULATIONS.
BASED ON THE IMAGE PROVIDED BELOW FOR THIS QUESTION:
- Calculate the amount of titrant used in each trial to reach endpoint.
- Report total hardness of the sample as mean ±sd.
a. 250.0 mL of 500.0 ppm of CaCO3 solution from a primary standard (assume solvent is distilled water only).
- Answer : Mass of CaCO3 = 0.125 g
b, The EDTA solution was standardized by titrating it with a 25.0 mL aliquot of the CaCO3 solution. How much of the titrant was consumed.
- Answer: Volume of EDTA consumed = 12.405 g
c. Calculate the average titer (mg CaCO3/mL EDTA).
- Mass of CaCO3 in 25 mL CaCO3 solution: 0.0125 g
- Answer: 1.008 mg CaCO3/ mL EDTA
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images




