What is the immediate Gibbs free energy change for the reaction? In what direction the reaction will spontaneously approach equilibrium? Enter your answer in the box provided with correct units and sig
What is the immediate Gibbs free energy change for the reaction? In what direction the reaction will spontaneously approach equilibrium? Enter your answer in the box provided with correct units and sig
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section5.7: Moleculat Speeds; Diffusion And Effusion
Problem 5.6CC
Related questions
Question
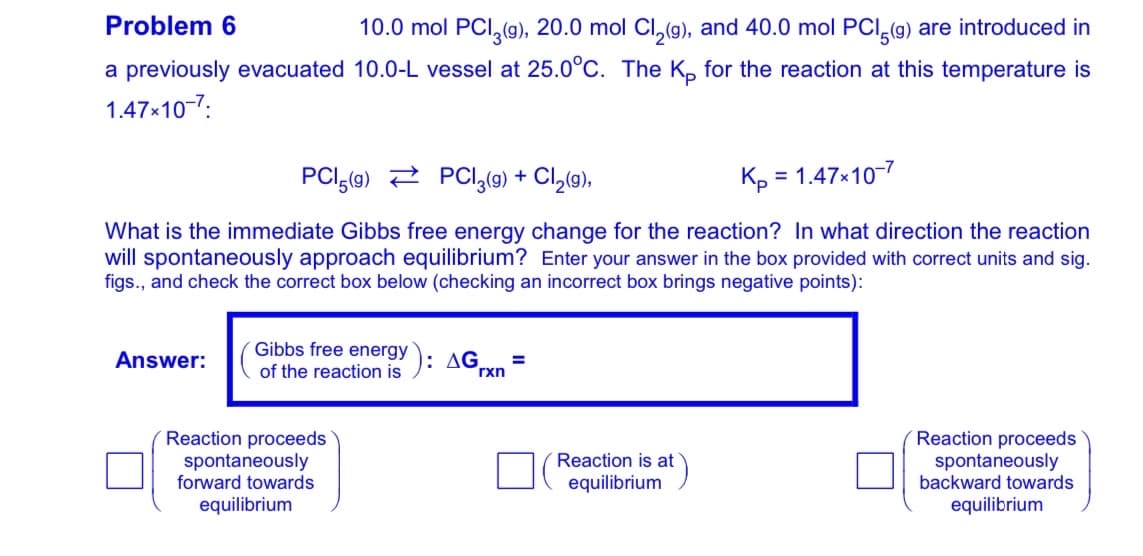
Transcribed Image Text:Problem 6
10.0 mol PCI, (g), 20.0 mol Cl, (g), and 40.0 mol PCI,(g) are introduced in
a previously evacuated 10.0-L vessel at 25.0°C. The K, for the reaction at this temperature is
1.47x10-7:
PCI,(9) 2 PCI,(g9) + Cl,(9),
= 1.47x10-7
What is the immediate Gibbs free energy change for the reaction? In what direction the reaction
will spontaneously approach equilibrium? Enter your answer in the box provided with correct units and sig.
figs., and check the correct box below (checking an incorrect box brings negative points):
Gibbs free energy
Answer:
): AGrxn
of the reaction is
Reaction proceeds
spontaneously
forward towards
Reaction proceeds
spontar
sly
backward towards
Reaction is at
equilibrium
equilibrium
equilibrium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning