Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.25QAP
Related questions
Question
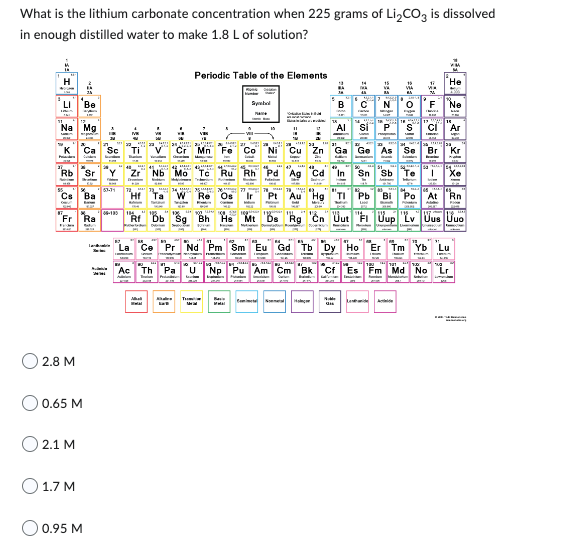
Transcribed Image Text:What is the lithium carbonate concentration when 225 grams of Li₂CO3 is dissolved
in enough distilled water to make 1.8 L of solution?
H
LI Be
Na Mg
Rb Sr
Cs Ba
B
Fr
2.8 M
O 0.65 M
2.1 M
Ca Sc
O 1.7 M
0.95 M
Ra
53-31
Ti
Ac
Hf
EMER
Cr Mn Fe
Periodic Table of the Elements
W Re
Zr Nb Mo Tc Ru Rh Pd Ag Cd
Symbol
Pa
Co Ni Cu
T
Pt
Rf Db Sg Bh Hs Mt Ds Rg Cn Uut "FI
ped
Ce Pr Nd
Nd Pm Sm Eu Gd
Zn
Au Hg Tl Pb
B
Holger
Hadde
sa za
VIA
Bi Po
Uup "Lv
VA
He
Ne
Ar
Dy Ho
Lu
Np Pu Am Cm Bk Cf Es Fm Md No Lr
At Rn
Uus Juo
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning