What is the molality of a solution prepared by dissolving 78.8 g of ethylene glycol, HOCH2CH2OH, in 1.45 L of water? Assume the density of water is 1.00 g/mL
What is the molality of a solution prepared by dissolving 78.8 g of ethylene glycol, HOCH2CH2OH, in 1.45 L of water? Assume the density of water is 1.00 g/mL
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section: Chapter Questions
Problem 104SCQ: Sodium chloride (NaCl) is commonly used to melt ice on roads during the winter. Calcium chloride...
Related questions
Question
1) What is the molality of a solution prepared by dissolving 78.8 g of ethylene glycol, HOCH2CH2OH, in 1.45 L of water? Assume the density of water is 1.00 g/mL.
2)The concentrations of Al and K in a sample of riverwater are 0.0500 mg/kg and 1.30 mg/kg, respectively. Express the concentration in molality.
3)
What molality of nonvolatile solute is needed to lower the melting point of camphor by 1.050°C? (Kf = 39.70ºC/m)
4) Determine the melting point of an aqueous solution containing 193 mg of saccharin (C7H5O3NS) added to 1.00 mL of water (density of water = 1.00 g/mL, Kf = 1.86°C/m).
5) Which one of the following aqueous solutions should have the lowest freezing point?
a. 0.0150 m Na2SO4
b. 0.0300 m KBr
c. 0.00500 m C6H12O6
5) From your predicted value of the van't Hoff factor, calculate the boiling point of a 1.64 m aqueous solution of barium nitrate, Ba(NO3)2. The Kb of water is 0.52°C/m.
Expert Solution
Step 1
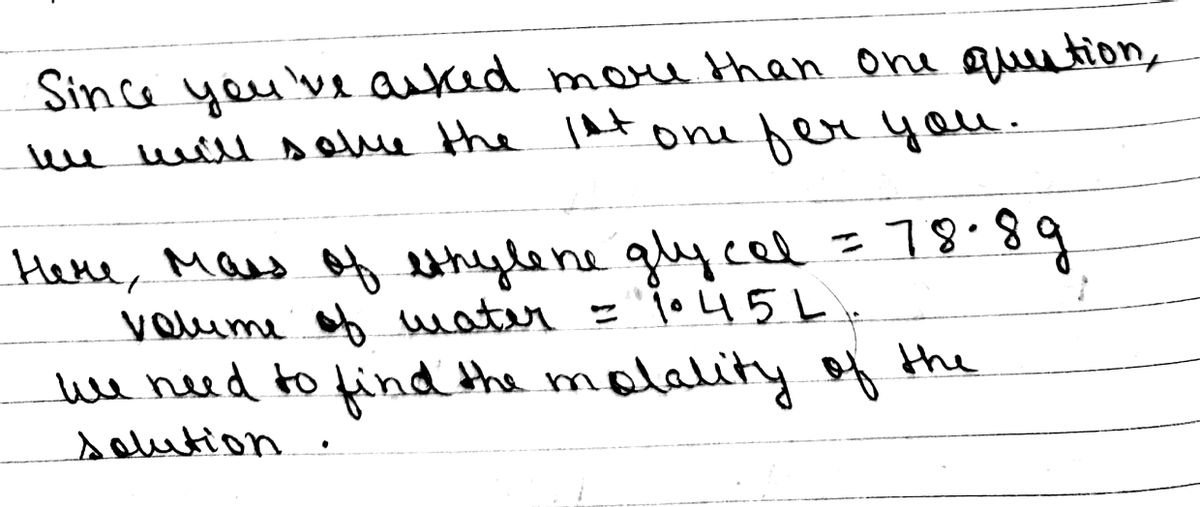
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning