. What is the weight of the sample in trial 1? 2. What is the average weight of the precipitate in trial 1? 3. From the computed weight of the sample in trial 1, what is the theoretical amount of silver nitrate solution required to precipitate all of the chloride ions as silver chloride? 4. What is the molecular weight of the precipitate?
. What is the weight of the sample in trial 1? 2. What is the average weight of the precipitate in trial 1? 3. From the computed weight of the sample in trial 1, what is the theoretical amount of silver nitrate solution required to precipitate all of the chloride ions as silver chloride? 4. What is the molecular weight of the precipitate?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
100%
1. What is the weight of the sample in trial 1?
2. What is the average weight of the precipitate in trial 1?
3. From the computed weight of the sample in trial 1, what is the theoretical amount of silver nitrate solution required to precipitate all of the chloride ions as silver chloride?
4. What is the molecular weight of the precipitate?
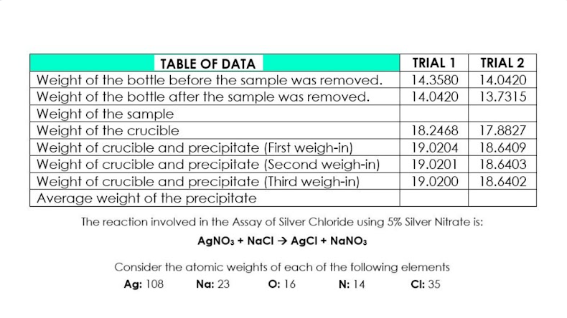
Transcribed Image Text:TABLE OF DATA
TRIAL 1
TRIAL 2
Weight of the bottle before the sample was removed.
Weight of the bottle after the sample was removed.
Weight of the sample
Weight of the crucible
Weight of crucible and precipitate (First weigh-in)
Weight of crucible and precipitate (Second weigh-in)
Weight of crucible and precipitate (Third weigh-in)
Average weight of the precipitate
14.3580
14.0420
14.0420
13.7315
18.2468
19.0204
19.0201
19.0200
17.8827
18.6409
18.6403
18.6402
The reaction involved in the Assay of Silver Chloride using 5% Silver Nitrate is:
AGNO, + NacI > AgCI + NANO,
Consider the atomic weights of each of the following elements
Ag: 108
O: 16
Cl: 35
Na: 23
N: 14
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What is the percentage weight of the chloride ions in trial 1?
What is the percentage weight of the analyte in trial 1?
What is the gravimetric factor involved?*
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
