Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.105QE
Related questions
Question
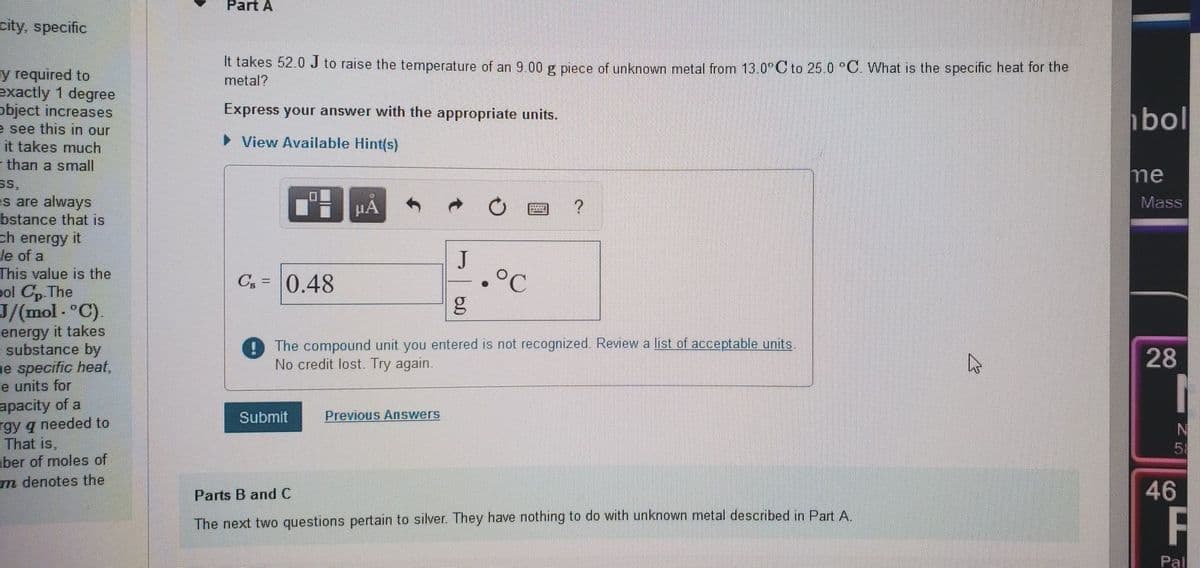
It takes 52.0 J to raise the temperature of an 9.00 g piece of unknown metal from 13.0∘C to 25.0 ∘C. What is the specific heat for the metal?
Express your answer with the appropriate units.
Why will not answer not be accepted?
Transcribed Image Text:city, specific
y required to
exactly 1 degree
object increases
e see this in our
it takes much
than a small
SS,
es are always
bstance that is
ch energy it
le of a
This value is the
Dol Cp.The
J/(mol. °C).
energy it takes
substance by
e specific heat,
e units for
apacity of a
gy a needed to
That is,
ber of moles of
m denotes the
Part A
It takes 52.0 J to raise the temperature of an 9.00 g piece of unknown metal from 13.0°C to 25.0 °C. What is the specific heat for the
metal?
Express your answer with the appropriate units.
► View Available Hint(s)
Cg =
0.48
µA
Submit
J
Previous Answers
PC
The compound unit you entered is not recognized. Review a list of acceptable units
No credit lost. Try again.
E
Parts B and C
The next two questions pertain to silver. They have nothing to do with unknown metal described in Part A.
hs
bol
me
Mass
28
51
46
F
Pall
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning