
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 44QAP
When ethanol (grain alcohol, 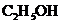 is burned in oxygen, approximately 1360 kJ of heat energy is released per mole of ethanol.
is burned in oxygen, approximately 1360 kJ of heat energy is released per mole of ethanol.
mg src=Images/HTML_99425-10-44QAP_image002.jpg alt="" align="top"/>
- What quantity of heat is released for each gram of ethanol burned?
i>What is 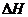 for the reaction as written?
for the reaction as written?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 10 Solutions
Introductory Chemistry: A Foundation
Ch. 10.1 - at if energy were not conserved? How would this...Ch. 10.4 - u are calculating in a chemistry problem. What if...Ch. 10.5 - ercise 10.1 How many calories of energy correspond...Ch. 10.5 - ercise 10.2 Calculate the joules of energy...Ch. 10.5 - ercise 10.3 A 5.63-g sample of solid gold is...Ch. 10.5 - ercise 10.4 A 2.8-g sample of pure metal requires...Ch. 10.6 - Prob. 10.5SCCh. 10.7 - at if Hess’s law were not true? What are some...Ch. 10.7 - Prob. 10.6SCCh. 10.9 - Prob. 1CT
Ch. 10.10 - at if the first law of thermodynamics was true,...Ch. 10 - Prob. 1ALQCh. 10 - friend of yours reads that the process of water...Ch. 10 - ou place hot metal into a beaker of cold water. ol...Ch. 10 - Prob. 4ALQCh. 10 - Prob. 5ALQCh. 10 - xplain why aluminum cans make good storage...Ch. 10 - n Section 10.7, two characteristics of enthalpy...Ch. 10 - Prob. 8ALQCh. 10 - hat is meant by the term driving forces? Why are...Ch. 10 - Prob. 10ALQCh. 10 - Explain in your own words what is meant by the...Ch. 10 - Prob. 12ALQCh. 10 - What if energy was not conserved? How would this...Ch. 10 - The internal energy of a system is said to be the...Ch. 10 - Hydrogen gas and oxygen gas react violently to...Ch. 10 - Consider four 100.0-g samples of water, each in a...Ch. 10 - For each of the following situations ac. use the...Ch. 10 - Prob. 18ALQCh. 10 - Does the entropy of the system increase or...Ch. 10 - Prob. 20ALQCh. 10 - Prob. 1QAPCh. 10 - Prob. 2QAPCh. 10 - Prob. 3QAPCh. 10 - Prob. 4QAPCh. 10 - Prob. 5QAPCh. 10 - n Fig. 10.1, what kind of energy does ball A...Ch. 10 - Prob. 7QAPCh. 10 - f you spilled a cup of freshly brewed hot tea on...Ch. 10 - Prob. 9QAPCh. 10 - Prob. 10QAPCh. 10 - In studying heat flows for chemical processes,...Ch. 10 - When a chemical system evolves energy, where does...Ch. 10 - The combustion of methane, is an exothermic...Ch. 10 - Are the following processes exothermic or...Ch. 10 - What do we mean by thermodynamics? What is the...Ch. 10 - Prob. 16QAPCh. 10 - Prob. 17QAPCh. 10 - If q for a process is a positive number, then the...Ch. 10 - For an endothermic process, q will have a...Ch. 10 - A system absorbs 215 kJ of heat, and 116 kJ of...Ch. 10 - Prob. 21QAPCh. 10 - Prob. 22QAPCh. 10 - If 8.40 kJ of heat is needed to raise the...Ch. 10 - If it takes 654 J of energy to warm a 5.51-g...Ch. 10 - Prob. 25QAPCh. 10 - Prob. 26QAPCh. 10 - Covert the following numbers of kilojoules into...Ch. 10 - Prob. 28QAPCh. 10 - Prob. 29QAPCh. 10 - Prob. 30QAPCh. 10 - .5 kJ of heat is applied to a 1012-g block of...Ch. 10 - What quantity of heat energy must have en applied...Ch. 10 - If 125 J of heat energy is applied to a block of...Ch. 10 - If 100. J of heat energy is applied to a 25-g...Ch. 10 - What quantity of heat is required to raise the...Ch. 10 - Prob. 36QAPCh. 10 - The “Chemistry in Focus” segment Nature Has Hot...Ch. 10 - In the “Chemistry in Focus” segment Firewalking:...Ch. 10 - Prob. 39QAPCh. 10 - A _________ is a device used to determine the heat...Ch. 10 - The enthalpy change for the reaction of hydrogen...Ch. 10 - For the reaction kJ per mole of formed. Calculate...Ch. 10 - Prob. 43QAPCh. 10 - When ethanol (grain alcohol, is burned in oxygen,...Ch. 10 - Prob. 45QAPCh. 10 - Prob. 46QAPCh. 10 - Prob. 47QAPCh. 10 - Prob. 48QAPCh. 10 - Prob. 49QAPCh. 10 - Prob. 50QAPCh. 10 - Prob. 51QAPCh. 10 - Prob. 52QAPCh. 10 - Prob. 53QAPCh. 10 - Prob. 54QAPCh. 10 - Prob. 55QAPCh. 10 - Prob. 56QAPCh. 10 - Prob. 57QAPCh. 10 - Prob. 58QAPCh. 10 - Prob. 59QAPCh. 10 - Prob. 60QAPCh. 10 - If a reaction occurs readily but has an...Ch. 10 - Prob. 62QAPCh. 10 - Prob. 63QAPCh. 10 - Prob. 64QAPCh. 10 - Which of the following is an endothermic process?...Ch. 10 - Prob. 66APCh. 10 - Prob. 67APCh. 10 - Calculate the amount of energy required (in...Ch. 10 - If takes 1.25 kJ of energy to heat a certain...Ch. 10 - What quantity of heat energy would have to be...Ch. 10 - The specific heat capacity of gold is 0.13 J/g °C....Ch. 10 - Calculate the amount of energy required (in...Ch. 10 - If 10. J of heat is applied to 5.0-g samples of...Ch. 10 - A 50.1)-g sample of water at 100. °C is poured...Ch. 10 - A 25.0-g sample of pure iron at 85 °C is dropped...Ch. 10 - If 7.24 kJ of heat is applied to a 952-g block of...Ch. 10 - For each of the substances listed in Table 10.1,...Ch. 10 - A system releases 213 kJ of heat and has a...Ch. 10 - Prob. 79APCh. 10 - Calculate the enthalpy change when 5.00 g of...Ch. 10 - Prob. 81APCh. 10 - Prob. 82APCh. 10 - It has been determined that the body can generate...Ch. 10 - Prob. 84APCh. 10 - Prob. 85CPCh. 10 - The specific heat capacity of graphite is 0.71 J/g...Ch. 10 - A swimming pool, 10.0 in by 4.0 m, is filled with...Ch. 10 - Prob. 88CPCh. 10 - Prob. 89CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Although the gas used in an oxyacetylene torch (Figure 5.7) is essentially pure acetylene, the heat produced by combustion of one mole of acetylene in such a torch is likely not equal to the enthalpy of combustion of acetylene listed in Table 5.2. Considering the conditions for which the tabulated data are reported, suggest an explanation.arrow_forwardWhen one mol of KOH is neutralized by sulfuric acid, q=56 kJ. (This is called the heat of neutralization.) At 23.7C, 25.0 mL of 0.475 M H2SO4 is neutralized by 0.613 M KOH in a coffee-cup calorimeter. Assume that the specific heat of all solutions is 4.18J/gC, that the density of all solutions is 1.00 g/mL, and that volumes are additive. (a) How many mL of KOH is required to neutralize H2SO4? (b) What is the final temperature of the solution?arrow_forwardA 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 19.6C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, the specific heat of all solutions is the same as that of water, and volumes are additive.arrow_forward
- A 29.1-mL sample of 1.05 M KOH is mixed with 20.9 mL of 1.07 M HBr in a coffee-cup calorimeter (see Section 6.6 of your text for a description of a coffee-cup calorimeter). The enthalpy of the reaction, written with the lowest whole-number coefficients, is 55.8 kJ. Both solutions are at 21.8C prior to mixing and reacting. What is the final temperature of the reaction mixture? When solving this problem, assume that no heat is lost from the calorimeter to the surroundings, the density of all solutions is 1.00 g/mL, and volumes are additive.arrow_forwardMagnesium sulfate is often used in first-aid hot packs, giving off heat when dissolved in water. A coffee-cup calorimeter at 25C contains 15.0 mL of water at 25C. A 2.00-g sample of MgSO4 is dissolved in the water and 1.51 kJ of heat are evolved. (You can make the following assumptions about the solution: volume=15.0 mL, density=1.00 g/mL, specific heat=4.18J/gC.) (a) Write a balanced equation for the solution process. (b) Is the process exothermic? (c) What is qH2O? (d) What is the final temperature of the solution? (e) What are the initial and final temperatures in F?arrow_forwardPeanuts and peanut oil are organic materials and bum in air. How many burning peanuts does it take to provide the energy to boil a cup of water (250 mL of water)? To solve this problem, we assume each peanut, with an average mass of 0.73 g, is 49% peanut oil and 21% starch; the remainder is noncombustible We further assume peanut oil is palmitic acid, C16H32O2, with an enthalpy of formation of 848.4 kJ/mol. Starch is a long chain of C6H10O5 units, each unit having an enthalpy of formation of 960 kJ.arrow_forward
- In the “Chemistry in Focus” segment Firewalking: Magic or Science?, it is claimed that one reason people can walk on hot coals is that human tissue is mainly composed of water. Because of this, a large amount of heat must be transferred from the coals to change the temperature of the feet significantly. How much heat must be transferred to 100.0 g of water to change its temperature by 35 °C?arrow_forward9.104 An engineer is using sodium metal as a cooling agent in a design because it has useful thermal properties. Looting up the heat capacity, the engineer finds a value of 28.2 J mol-l °C-l. Carelessly, he wrote this number down without units. As a result, it was later taken as specific heat. (a) What would he the difference between these two values? (b) Would the engineer overestimate the ability of sodium to remove heat from the system or underestimate it because of this error? Be sure to explain your reasoning.arrow_forwardHow many moles of isooctane must be burned to produce loo U of heat under standard state conditions?arrow_forward
- From the data in Table 5.2, determine which of the following fuels produces the greatest amount of heat per gram when burned under standard conditions: CO(g) , CH4(g) , or C2H2(g) .arrow_forwardConsider the combustion of propane: C3H8(g)+5O2(g)3CO2(g)+4H2O(l)H=2221KJ Assume that all the heat in Example 7-3 comes from the combustion of propane. What mass of propane must be burned to furnish this amount of energy assuming the heat transfer process is 60.% efficient?arrow_forwardIn the 1880s, Frederick Trouton noted that the enthalpy of vaporization of 1 mol pure liquid is approximately 88 times the boiling point, Tb, of the liquid on the Kelvin scale. This relationship is called Troutons rule and is represented by the thermochemical equation liquid gas H = 88 Tb, joules Combined with an empirical formula from chemical analysis, Troutons rule can be used to find the molecular formula of a compound, as illustrated here. A compound that contains only carbon and hydrogen is 85.6% C and 14.4% H. Its enthalpy of vaporization is 389 J/g, and it boils at a temperature of 322 K. (a) What is the empirical formula of this compound? (b) Use Troutons rule to calculate the approximate enthalpy or vaporization or one mole of the compound. Combine the enthalpy of vaporization per mole with that same quantity per gram to obtain an approximate molar mass of the compound. (c) Use the results of parts (a) and (b) to find the molecular formula of this compound. Remember that the molecular mass must be exactly a whole-number multiple of the empirical formula mass, so considerable rounding may be needed.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY