Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 80QAP: In the photoelectric effect, electrons are ejected from a metal surface when light strikes it. A...
Related questions
Question
answer 10min
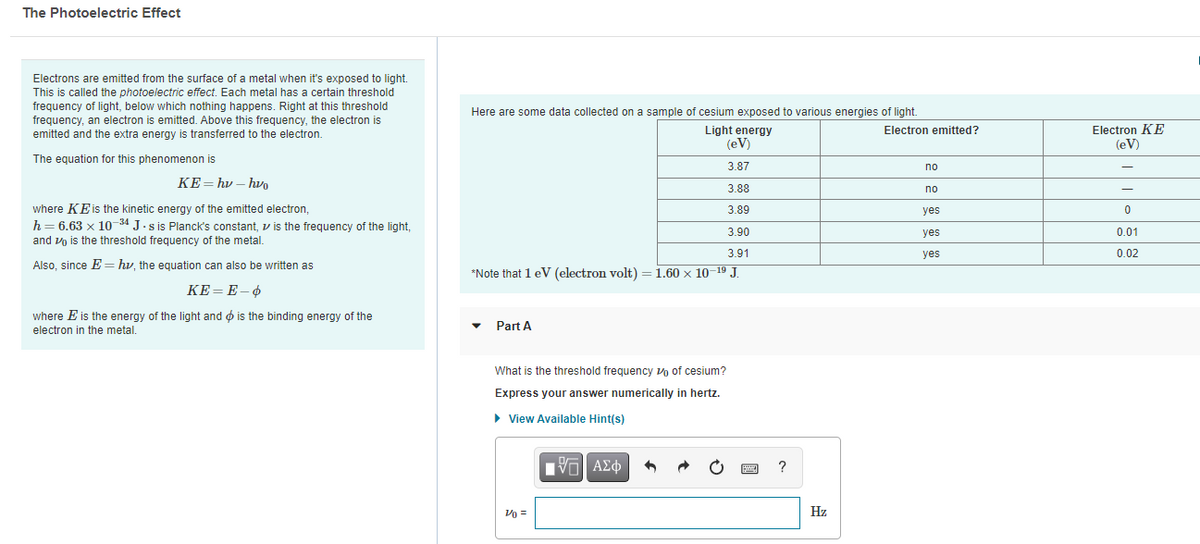
Transcribed Image Text:The Photoelectric Effect
Electrons are emitted from the surface of a metal when it's exposed to light.
This is called the photoelectric effect. Each metal has a certain threshold
frequency of light, below which nothing happens. Right at this threshold
frequency, an electron is emitted. Above this frequency, the electron is
emitted and the extra energy is transferred to the electron.
Here are some data collected on a sample of cesium exposed to various energies of light.
Light energy
(eV)
Electron KE
(eV)
Electron emitted?
The equation for this phenomenon is
3.87
no
KE= hv – hv
3.88
no
where KEis the kinetic energy of the emitted electron,
h= 6.63 x 10 34 J.s is Planck's constant, v is the frequency of the light,
3.89
yes
3.90
yes
0.01
and vo is the threshold frequency of the metal.
3.91
yes
0.02
Also, since E== hv, the equation can also be written as
*Note that 1 eV (electron volt) = 1.60 x 10–19 J.
КЕ-Е-Ф
where E is the energy of the light and ø is the binding energy of the
electron in the metal.
Part A
What is the threshold frequency vo of cesium?
Express your answer numerically in hertz.
View Available Hint(s)
ν ΑΣφ
?
Vo =
Hz
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning