Q: makes it difficult to compare Kp values directly. HgS Kp 1.6 x 104 Cus 8.5 x 1045 Ag:S 1.6 x 10 BizS…
A: Ksp : At normal temperature, if the solubility of a salt is 0.1mol / Lit then that salt is called…
Q: Determine the equilibrium constant for a reaction at 230.0 K if ∆G° =-13.70 kJ/mol. (R = 8.314 J/mol…
A: The relation between standard Gibbs free energy change(ΔG°) and equilibrium constant(Keq) is given…
Q: The equilibrium constant for the reaction AgBr(s) Ag+(aq) + Br– (aq) is the solubility product…
A: Equilibrium Constant (Kc) expression for the given reaction is as follows:
Q: Which of the following is correct concerning the following system at equilibrium at 298 K? A +B=C+ D…
A:
Q: The equilibrium constant for the reaction Naz(g) - 2Nalg) is 2.47 at 1000. K. Calculate the value of…
A:
Q: The Kp for the reaction A (g) ⇌ 2 B (g) is 0.0270. What is Kp for the reaction 4 B (g) ⇌ 2 A (g)?
A: The reaction given is A (g) ⇌ 2 B (g) => Kp1 = [B]2 / [A] = 0.0270 where [A] = equilibrium…
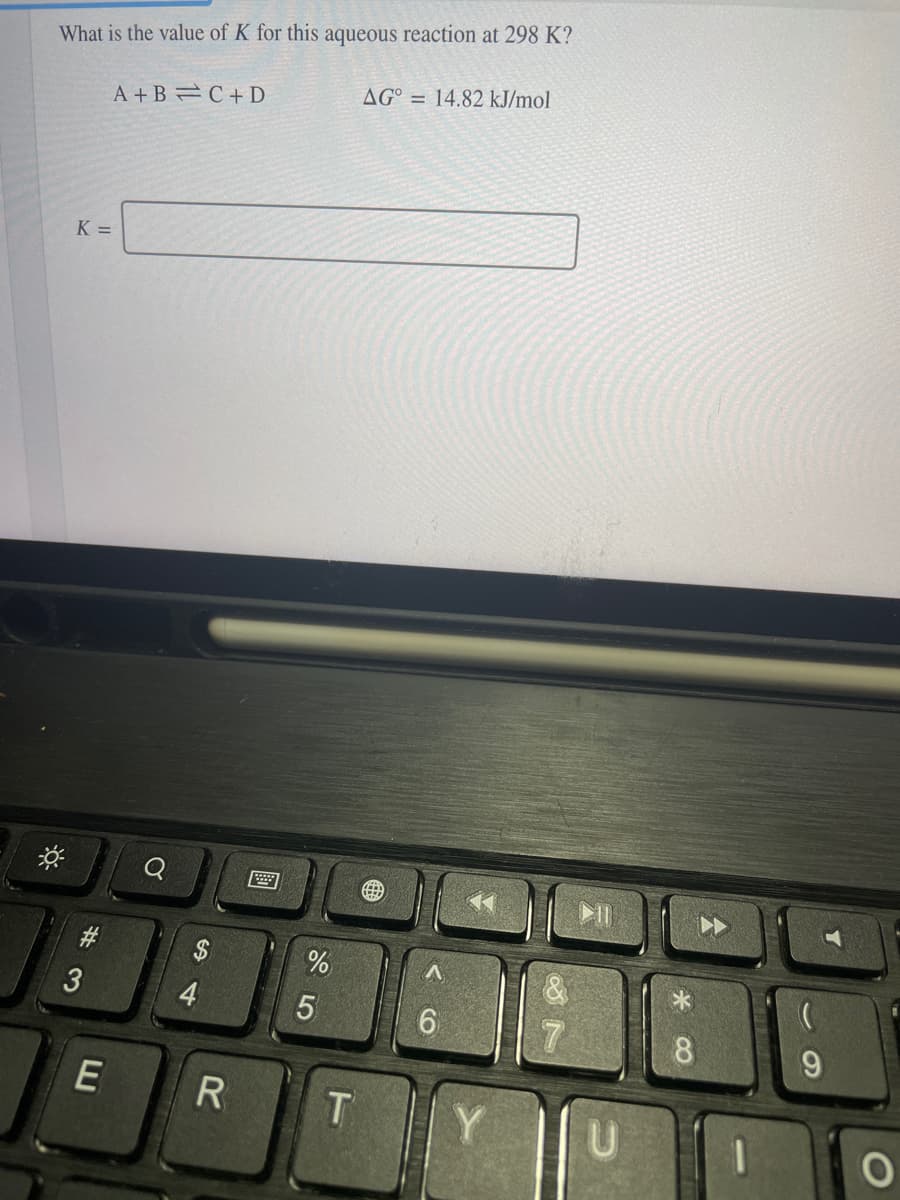
Q: What is the value of K for this aqueous reaction at 298 K? A + B =C+D AG° = 26.27 kJ/mol K =
A: You can see Relation between them, ∆G° = -RTln(K) Where : ln(K) = 2.303 log(K) Apply this, You…
Q: Determine AG° for the reaction N2O4(g) = 2 NO2(g) (K= 0.144 at 298 K).
A: Given :- K = 0.144 T = 298 K To be calculated :- ∆G° (in kJ/mol) Formula :- ∆G° =…
Q: Calculate Ksp for the ff. substances, given the molar concentration of their saturated solution.…
A:
Q: What is the value of K for this aqueous reaction at 298 K? A + B =C+ D AG° = 12.16 kJ/mol K =
A:
Q: The equilibrium constant for the reaction N204 (g) + 2 NO2 (g) is 0.100 at 45 °C. Calculate AG° for…
A:
Q: Consider the reaction at 298 K: A+B-C The equilibrium constant for the reaction is 23. If the…
A: To determine the gibbs free energy for a reaction we use the formula- Option (4) is correct [ -347…
Q: 4. Calculate the equilibrium constant for the reaction below: Nzig)t Oz(g) + Br2(g) = 2NOB (g) Kp =?…
A: Multiplying reaction 1 given with a constant 2 gives 2 NO (g) + Br2 (g) ----> 2 NOBr…
Q: 6) What is the relationship between Kp and Kc for the reaction: H2(g) + I2(@) 2Hl(9)
A:
Q: Determine the equilibrium constant K for the following reaction at 298 K. Cl(g) + O3(g) - CIO(g) +…
A:
Q: Calculate the equilibrium constant (K) for the following reaction? 2Fe³+ + 31 2Fe²+ 2Fe²+ + 13…
A:
Q: 1) The following reaction was allowed to reach equilibrium at 25oC. Enclosed with the phase of each…
A: Since you have asked two question, we will solve first one for you. If you want other question to be…
Q: The Kp for the reaction A (g) ⇌ 2 B (g) is 0.0450. What is Kp for the reaction 4 B (g) ⇌ 2 A (g)?
A: Given, A(g) ⇌ 2 B(g) Kp= 0.0450 4 B(g) ⇌ 2 A(g) Kp’= ?
Q: 6. Find AG and AH for the following reaction. Find K at 298K. Fe2O3 (s) + H2 (g) Fe;O4 (s) + H20 (g)
A: Here we have to determine ∆G° and ∆H° and equilibrium constant at 298 K of the following given…
Q: The Kp for the reaction A (g) ⇌ 2 B (g) is 0.0310. What is Kp for the reaction 4 B (g) ⇌ 2 A (g)?
A:
Q: The equilibrium constant, Kc, for the following reaction is 1.80x10-2 at 698 K. Calculate Kp for…
A: Relation between Kp and Kc is Kp = Kc (RT)∆ng where ∆ng = No.of moles of gaseous products -…
Q: Determine the equilibrium constant for a reaction at 260.0 K if ∆G° =21.20 kJ/mol. (R = 8.314 J/mol…
A: Given: Temperature is 260 K ∆G° is 21.20 kJ/mol Gas constant, R is 8.314 J/mol. K To find:…
Q: The reaction between iodide ion, I, and I, to form triiodide ion, I;", is shown below. Calculate AG°…
A: Kc is the equilibrium constant at concentration C. ∆Go is the standard Gibbs free energy. Data…
Q: What is the value of K for this aqueous reaction at 298 K? A +B =C+D AG° = 29.10 kJ/mol K =
A:
Q: kı, 2NOC1(g) k.1 ki = 1.0x10° M:'s' and k-1 = 2.0 M-'s'. For reaction 2NO(g) + Cl2 (g) 5) What is…
A: Equilibrium constant can be determined using the given formula,
Q: The vapor pressure of water at 25oC is 0.0313 atm. Calculate the value of Kc at 25oC for the…
A:
Q: Which is the correct expression for K. for this reaction? Fe203 (s) + 3 CO (g) 2 Fe (e) + 3 CO2 (g)
A: Given chemical reaction is: Fe2O3(s) + 3CO(g) ----> 2Fe(l) + 3CO2(g) We need to determine…
Q: 4PC13 (1) 2. P4 (s) + 6C12 (g) Calculate the Kc for this reaction if Kp is equal to 3.76 x 10³ at…
A: Dear student , since you have posted multiple questions we are allowed to solve only first…
Q: The equilibrium constant Kc equals 0.0952 for the following reaction at 227°C.CH3OH(g) _ CO(g) +…
A: Since the relationship between Kc and Kp for any reaction is given by Kp = Kc (RT)Δng where R =…
Q: 3. Calculate the equilibrium constant for the following reaction at 298 K. 3C2H2 (g) + CeHe (g) AG°…
A:
Q: Enter your answer in the provided box. Calculate the Kp for the following reaction at 25°C: H2(g) +…
A: The balanced reaction taking place is given as, => H2 (g) + I2 (g) ----> 2 HI (g) Given : ΔGo…
Q: The equilibrium constant for the following reaction is 4.0 x 10° at 25 °C N2 (g) + 3H2 (g) 2NH3 (g)…
A:
Q: Express the equilibrium constant for the following reaction. RbBrO3 (s) = RbBrO(s) + O2(g)
A: Given :- RbBrO3(s) <-----> RbBrO(s) + O2(g) To write :- equilibrium constant expression for…
Q: For the following equilibrium at 298 K, the equilibrium constant K = 2.84 x 1022 M5. Ag;PO4(s) +…
A: Natural reactions are two types- (a) irreversible reaction (b) reversible reaction A chemical…
Q: The Kp for the reaction A (g) =2 B (g) is 0.0450. What is Kp for the reaction 4 B (g) =2 A (g)?
A: Consider the given equilibrium reaction as; A g ⇌ 2 B g…
Q: What is the equilibrium constant for a reaction at 25 °C. The value of AG is - 57.5 kJ/mol. (R-8.315…
A: Gibbs' free energy : It is used to calculate the maximum reversible work that can be done by a…
Q: Calculate the values of Kp and Kc for the system at 525 °C, with the equilibrium pressure of CO2 at…
A: For a general reversible reaction xA(g) ⇔yB(g) The expression of Kc is Kc = [ B ]y / [ A ]x [ B…
Q: 26. For each reaction, an equilibrium constant at 298 K is given. Calculate AG° for each reaction.…
A:
Q: What is the value of K for this aqueous reaction at 298 K? A +B C+ D AG° = 26.25 kJ/mol K =
A: At standard conditions: ∆G0 = -RTlnK K----->>> Equilibrium constant R------>> Gas…
Q: What is the value of K for this aqueous reaction at 298 K? A + B C + D AG° = 25.80 kJ/mol K =
A: Given datas : T = 298 K ∆G° = 25.80 kJ/mol = 25.80 × 103 J/mol K = ? A + B <---> C + D
Q: The equilibrium constant for the reaction N204 (g) =* 2 NO2 (g) is 0.200 at 65 °C. Calculate AG° for…
A: Given :- N2O4(g) <-----> 2NO2(g) K = 0.200 T = 65°C To calculate :- ∆G° for this reaction
Q: The Kp for the reaction A (g)=2 B (g) is 0.0830. What is Kp for the reaction 2 A (g)=4 B (g)?
A: The given chemical equation is as follows: A(g) ⇌ 2B(g) Kp=0.0830 2A(g) ⇌ 4B(g) Kp'=?
Q: Determine AG° and the spontaneity of the reaction A2(g) + 3 X2(g) → 2 AX3(g) at 591 K. AH° = -86…
A: Since, ∆G°=∆H°-T∆S° where, ∆G°=standard change in Gibb's free energy ∆H°=standard…
Q: The Kp for the reaction A (g) ⇌ 2 B (g) is 0.0970. What is Kp for the reaction 2 A (g) ⇌ 4 B (g)?
A: Equilibrium constant :- It is expressed as the ratio of product to the reactant.
Q: What is the equilibrium constant at 1000 K for the reaction shown below if its AG at 1000 K is 32…
A:
Q: what is ΔG for a certain reaction of 300 degrees Kelvin if Kp is 6.0 x 10^2
A: Given-> T = 300 K Kp = 6.0 × 102
Q: The Kp for the reaction A (g) ⇌ 2 B (g) is 0.0620. What is Kp for the reaction 4 B (g) ⇌ 2 A (g)?
A: The equilibrium constant in terms of partial pressure (Kp) expresses the relationship between…
Q: What is the value of K for this aqueous reaction at 298 K? A+B↽−−⇀C+D Δ?°=10.14 kJ/mol…
A: Interpretation- To determine the value of K for this aqueous reaction at 298 K , which is given as…
Q: Calculate the equilibrium constant (Kc) for the given reaction. Kp = 0.003 and T = 298 K 2C0e) +…
A: The equilibrium constant with respect to concentration (KC) is related to equilibrium constant with…
The Gibbs free energy and the equilibrium constant are related by the following equation:
Where ∆Go = change in Gibbs free energy = 14.82 kJ/mol
R = gas constant =8.314 J.K-1.mol-1
T = absolute temperature = 298 K
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

- Calculate the Gibbs free energy of the reaction from mixing 50 mL each of 0.50 M Ag+(aq) solution and 1 M of NaCl(aq) to form AgCl(s) at 25°. The Ksp of AgCl is 6.0 × 10—11 at 25 °C.Calculate the Gibbs free energy of the reaction from mixing 50 mL each of 0.50 M AgNO3(aq) solution and 1 M of NaCl(aq) to form AgCl(s) at 25°. The Ksp of AgCl is 6.0 × 10—11 at 25 °C.What is the value of K for this aqueous reaction at 298 K? A+B↽−−⇀C+D Δ?°=10.14 kJ/mol K= ____________
- Calculate the value of the equilibrium constant, Kc, for the reaction H2(g) + 1/2 S2(g) ßà H2S (g) at 283 K.1-What volume of 0.57 M NaCl solution contains 169.5 grams of NaCl? 2-Calculate the molality of a solution containing 175g of glucose (C6H12O6) in 1828 g of ethanol. 3- What volume of 0.87 N CaCO3 solution contains 183.9 grams of CaCO3? 7- How many Faraday is needed to deposit 5 moles of Gold (Au) from AuCl3 solution using electrolysis process. 8- Determine the oxidation number of the Chromium (Cr) in an unknown salt if electrolysis of a molten sample of this salt for 3 hours with a 15 amp current deposits 43.65 grams of Chromium metal at the cathode. 4-Oxidation number of C in H2CO3 isCalculate the Gibbs energy and equilibrium constant for the chemical reaction at a temperature of 298.15 K. Hg2Cl2(s) → → 2 Hg(l) +Cl2(g).
- The table below provides data for the enthalpy and entropy of formation of compounds A and B at standard conditions (298 K) Compound DHfo (kJ mol-1) Sfo(J K-1mol-1) A –135.2 189.2 B –157.6 192.1 i) Calculate the standard Gibbs energy of formation for A and B. ii) Calculate the Gibbs energy change for the reaction A - B at standard conditions. iii) Calculate the enthalpy change for the reaction A - B at 350 K if the average heat capacity C0p = 42 J.K-1.mol-1.give Full detailed and accurate solution solution. The calculation for the last delta S value is 47.62 J/KThe hardness of water (hardness count) is usually expressed in parts per million (by mass) of CaCO3. What is the molar concentration of Ca2+ ions in a water sample with a hardness count of 175mg CaCO3il? How many milliliters of concentrated sulfuric acid, 94.0% (w/w), specific gravity of 1.831 are required to prepare 1 liter of a 0.100 M solution? The solubility-product constant for Ce(IO3)3 is 3.2x1010. What is the Ce3+ concentration in a solution prepared by mixing 50.0 ml of 0.0250 M Ce3.. with 50.00 ml of water?
- Rose was studying the electric field effect on bacterial growth when he unknowingly carried out an anodic reaction on the platinum electrode that led to the discovery of the anticancer drug cisplatin, cis-[PtCl2(NH3)2]. > Determine the formation constant (Kf) of cisplatin at 25 °C.What is the value of K for this aqueous reaction at 298 K Delta G=28.66 A+B<---->C+DFor each of the following reactions, write a balancedequation, calculate the standard emf, calculate ΔG° at298 K, and calculate the equilibrium constant K at 298 K.(a) Aqueous iodide ion is oxidized to I2(s) by Hg22+ (aq).(b) In acidic solution, copper(I) ion is oxidized to copper(II)ion by nitrate ion. (c) In basic solution, Cr(OH)3(s) is oxidizedto CrO42-(aq) by ClO-(aq).

