What mass of barium nitrate, Ba(NO3)2 is needed to react with 0.0174 moles of phosphoric acid, H3PO4? 3 Ba(NO3)2(aq) + 2 H3PO4 → Bas(PO4)2(s) + 6 HNO3(aq) mol Ba(NO3)2 g Ba(NO3)2 mol HЗРО4 g НЗРО4 0.000266 0.0261 3.03 6.82
What mass of barium nitrate, Ba(NO3)2 is needed to react with 0.0174 moles of phosphoric acid, H3PO4? 3 Ba(NO3)2(aq) + 2 H3PO4 → Bas(PO4)2(s) + 6 HNO3(aq) mol Ba(NO3)2 g Ba(NO3)2 mol HЗРО4 g НЗРО4 0.000266 0.0261 3.03 6.82
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 63QAP: Consider the hypothetical reaction 8A2B3(s)+3X4(g)4A4X3(s)+12B2(g)When 10.0 g of A2B3(MM=225g/mol)...
Related questions
Question
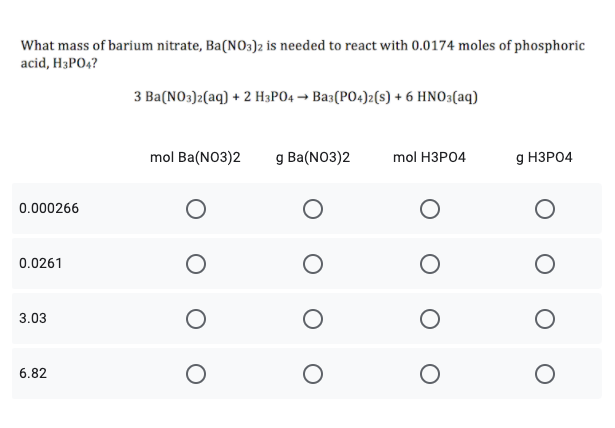
Transcribed Image Text:What mass of barium nitrate, Ba(NO3)2 is needed to react with 0.0174 moles of phosphoric
acid, H3PO4?
3 Ba(NO3)2(aq) + 2 H3PO4 → Bas(PO4)2(s) + 6 HNO3(aq)
mol Ba(NO3)2
g Ba(NO3)2
mol HЗРО4
g НЗРО4
0.000266
0.0261
3.03
6.82
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning