What mass of Cu(s) is electroplated by running 17.5 A of current through a Cu²“(aq) solution for 4.00 h ? Express your answer to three significant figures and include the appropriate units. Tmplates Symbols ueAdo redo resat keyboard shortcuts help. Value Units Request Answer Submit Part B How many minutes will it take to electroplate 22.1 g of gold by running 5.00 A of current through a solution of Au*(aq)? Express your answer to three significant figures and include the appropriate units.
What mass of Cu(s) is electroplated by running 17.5 A of current through a Cu²“(aq) solution for 4.00 h ? Express your answer to three significant figures and include the appropriate units. Tmplates Symbols ueAdo redo resat keyboard shortcuts help. Value Units Request Answer Submit Part B How many minutes will it take to electroplate 22.1 g of gold by running 5.00 A of current through a solution of Au*(aq)? Express your answer to three significant figures and include the appropriate units.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
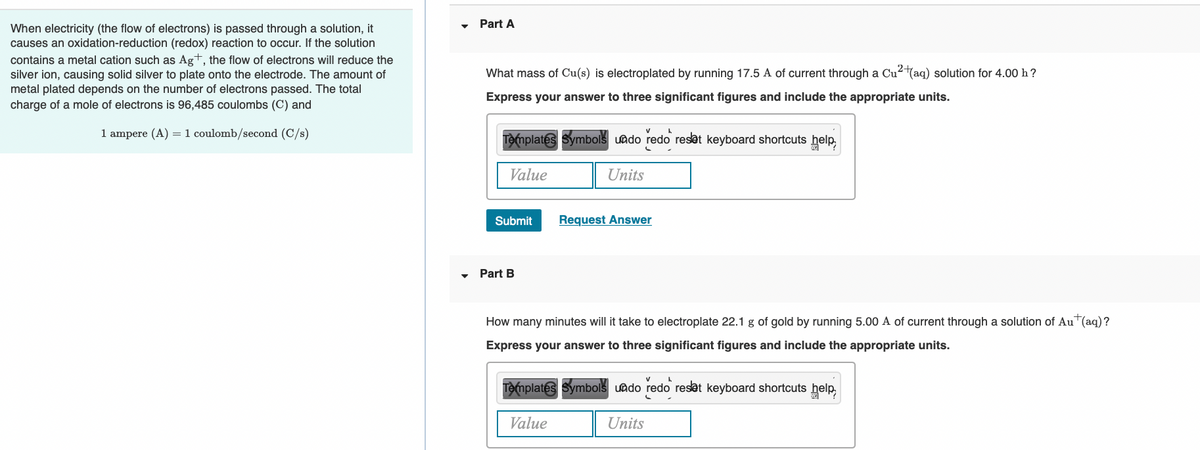
Transcribed Image Text:Part A
When electricity (the flow of electrons) is passed through a solution, it
causes an oxidation-reduction (redox) reaction to occur. If the solution
contains a metal cation such as Ag+, the flow of electrons will reduce the
silver ion, causing solid silver to plate onto the electrode. The amount of
What mass of Cu(s) is electroplated by running 17.5 A of current through a Cu2(aq) solution for 4.00 h?
metal plated depends on the number of electrons passed. The total
charge of a mole of electrons is 96,485 coulombs (C) and
Express your answer to three significant figures and include the appropriate units.
1 ampere (A) =1 coulomb/second (C/s)
Templates Symbols uado redo reset keyboard shortcuts help,
Value
Units
Submit
Request Answer
Part B
How many minutes will it take to electroplate 22.1 g of gold by running 5.00 A of current through a solution of AuT(aq)?
Express your answer to three significant figures and include the appropriate units.
Templates Symbols uado redo reset keyboard shortcuts
help,
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning