What mass of Fe(NO3)3 is needed to make 2.4 L of a 0.35 M solution? H LI Be Na Mg K Rb Sr Cs Ba Fr Ra 172.8 g 312.6 g 203.1 g 195.3 g 283.6 g 53-31 08-135 Periodic Table of the Elements Wwww EDWA Cr Mn Ti Zr Nb Mo "Hf Ta W Symbol Co Rf Db Sg Bh 15 Cu Tc Ru Rh Pd Ag Cd 79 35 26** Re Os "Pt Au Hg B La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho "Ac Th Pa U Np Pu Am Cm Bk Cf das P He FNe S CI Ar Se Br Kr Xe 150, Hs Mt Ds Rg Cn Uut "FI Uup Lv Uus Uuo Po At Rn Fm Md No Lu
What mass of Fe(NO3)3 is needed to make 2.4 L of a 0.35 M solution? H LI Be Na Mg K Rb Sr Cs Ba Fr Ra 172.8 g 312.6 g 203.1 g 195.3 g 283.6 g 53-31 08-135 Periodic Table of the Elements Wwww EDWA Cr Mn Ti Zr Nb Mo "Hf Ta W Symbol Co Rf Db Sg Bh 15 Cu Tc Ru Rh Pd Ag Cd 79 35 26** Re Os "Pt Au Hg B La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho "Ac Th Pa U Np Pu Am Cm Bk Cf das P He FNe S CI Ar Se Br Kr Xe 150, Hs Mt Ds Rg Cn Uut "FI Uup Lv Uus Uuo Po At Rn Fm Md No Lu
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 35CR
Related questions
Question
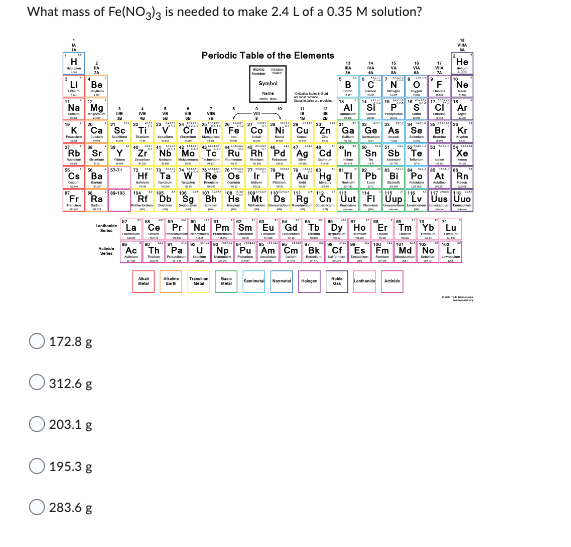
Transcribed Image Text:What mass of Fe(NO3)3 is needed to make 2.4 L of a 0.35 M solution?
H
LI
Be
Na Mg
K
Rb
Sr
Cs Ba
Fr Ra
172.8 g
312.6 g
203.1 g
195.3 g
283.6 g
53-31
08-135
Periodic Table of the Elements
Wwww EDWA
Cr Mn
Ti
Zr Nb Mo
35
"Hf Ta W Re
Tc
Rf Db Sg Bh
Symbol
Co
15
Cu
Cd
26
78.
Os "Pt Au Hg
Ru Rh Pd Ag
B
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho
"Ac Th Pa U Np Pu Am Cm Bk Cf
das
P
He
F Ne
S CI Ar
Se Br Kr
Xe
150,
Hs Mt Ds Rg Cn Uut "FI Uup Lv Uus Uuo
Po At Rn
Fm Md No
Lu
Ta
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning