What mass of the CuCl₂·2H₂O (170.48 g/mol) should be weighed in order to prepare the 250.0 mL Cu(II) (63.546 g/mol) stock solution? What is the mass of Cu in the unknown sample solution? What is the most probable identity of the unknown copper compound, assuming there is 1 mole of Cu per mole of the unknown?
What mass of the CuCl₂·2H₂O (170.48 g/mol) should be weighed in order to prepare the 250.0 mL Cu(II) (63.546 g/mol) stock solution? What is the mass of Cu in the unknown sample solution? What is the most probable identity of the unknown copper compound, assuming there is 1 mole of Cu per mole of the unknown?
Chapter27: Molecular Fluorescence Spectroscopy
Section: Chapter Questions
Problem 27.9QAP
Related questions
Question
What mass of the CuCl₂·2H₂O (170.48 g/mol) should be weighed in order to prepare the 250.0 mL Cu(II) (63.546 g/mol) stock solution?
What is the mass of Cu in the unknown sample solution?
What is the most probable identity of the unknown copper compound, assuming there is 1 mole of Cu per mole of the unknown?
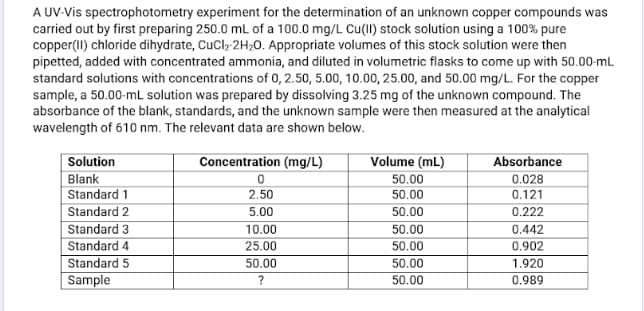
Transcribed Image Text:A UV-Vis spectrophotometry experiment for the determination of an unknown copper compounds was
carried out by first preparing 250.0 mL of a 100.0 mg/L Cu(II) stock solution using a 100% pure
copper(II) chloride dihydrate, CuCl, 2H20. Appropriate volumes of this stock solution were then
pipetted, added with concentrated ammonia, and diluted in volumetric flasks to come up with 50.00-mL
standard solutions with concentrations of 0, 2.50, 5.00, 10.00, 25.00, and 50.00 mg/L. For the copper
sample, a 50.00-mL solution was prepared by dissolving 3.25 mg of the unknown compound. The
absorbance of the blank, standards, and the unknown sample were then measured at the analytical
wavelength of 610 nm. The relevant data are shown below.
ITII
Solution
Concentration (mg/L)
Volume (mL)
Absorbance
Blank
50.00
0.028
Standard 1
2.50
50.00
0.121
Standard 2
5.00
50.00
0.222
Standard 3
Standard 4
10.00
50.00
0.442
25.00
50.00
0.902
Standard 5
50.00
50.00
1.920
Sample
50.00
0.989
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

