An aqueous Na;PO, solution is made using 42.3 g of Na,PO, diluted to a total solution volume of 2.00 L. 163.94 g Na3PO4) 1 mole Na3PO4 (molar mass a) In one calculation string, show the calculation for determining the molarity of the sodium phosphate solution. b) In one calculation string, show the calculation for determining the mass percent of the sodium phosphate solution from the molarity you determined in part a. c) Show the necessary calculations for determining the molality of sodium phosphate solution from the molarity you determined in part a. These calculations should be as condensed as possible, but don't need to be in one string. Assume a density of 1.08 g/mL for the solution.
An aqueous Na;PO, solution is made using 42.3 g of Na,PO, diluted to a total solution volume of 2.00 L. 163.94 g Na3PO4) 1 mole Na3PO4 (molar mass a) In one calculation string, show the calculation for determining the molarity of the sodium phosphate solution. b) In one calculation string, show the calculation for determining the mass percent of the sodium phosphate solution from the molarity you determined in part a. c) Show the necessary calculations for determining the molality of sodium phosphate solution from the molarity you determined in part a. These calculations should be as condensed as possible, but don't need to be in one string. Assume a density of 1.08 g/mL for the solution.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.104QE: A 10.00-mL sample of a 24.00% solution of ammonium bromide (NH4Br) requires 23.41 mL of 1.200 molar...
Related questions
Question
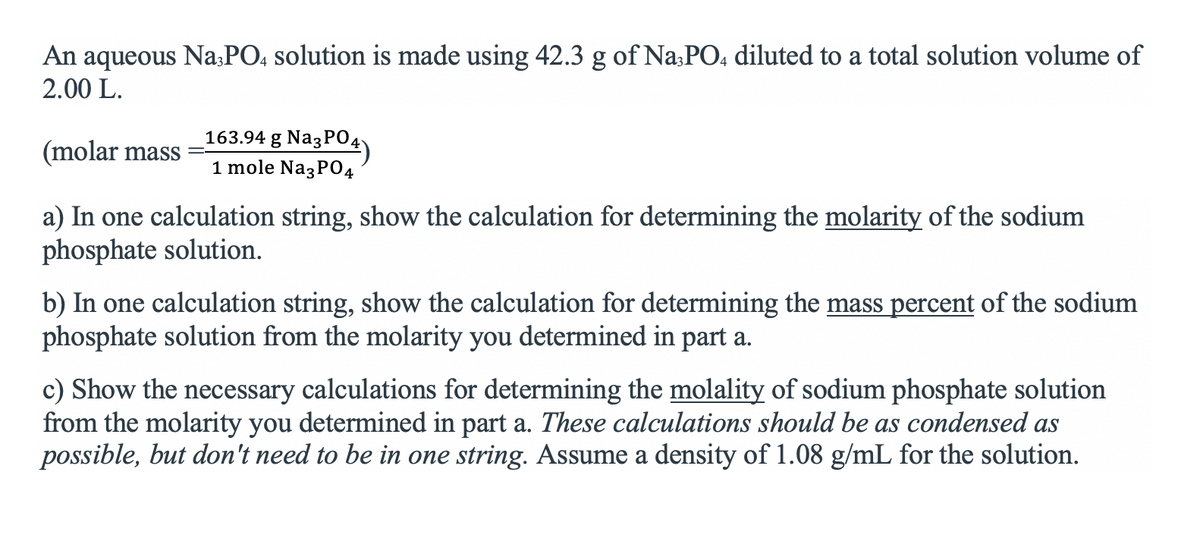
Transcribed Image Text:An aqueous Na;PO, solution is made using 42.3 g of Na:PO, diluted to a total solution volume of
2.00 L.
163.94 g NazPO4)
(molar mass
1 mole Na3P04
a) In one calculation string, show the calculation for determining the molarity of the sodium
phosphate solution.
b) In one calculation string, show the calculation for determining the mass percent of the sodium
phosphate solution from the molarity you determined in part a.
c) Show the necessary calculations for determining the molality of sodium phosphate solution
from the molarity you determined in part a. These calculations should be as condensed as
possible, but don't need to be in one string. Assume a density of 1.08 g/mL for the solution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning