Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter14: Mixtures And Solutions
Section: Chapter Questions
Problem 16STP
Related questions
Question
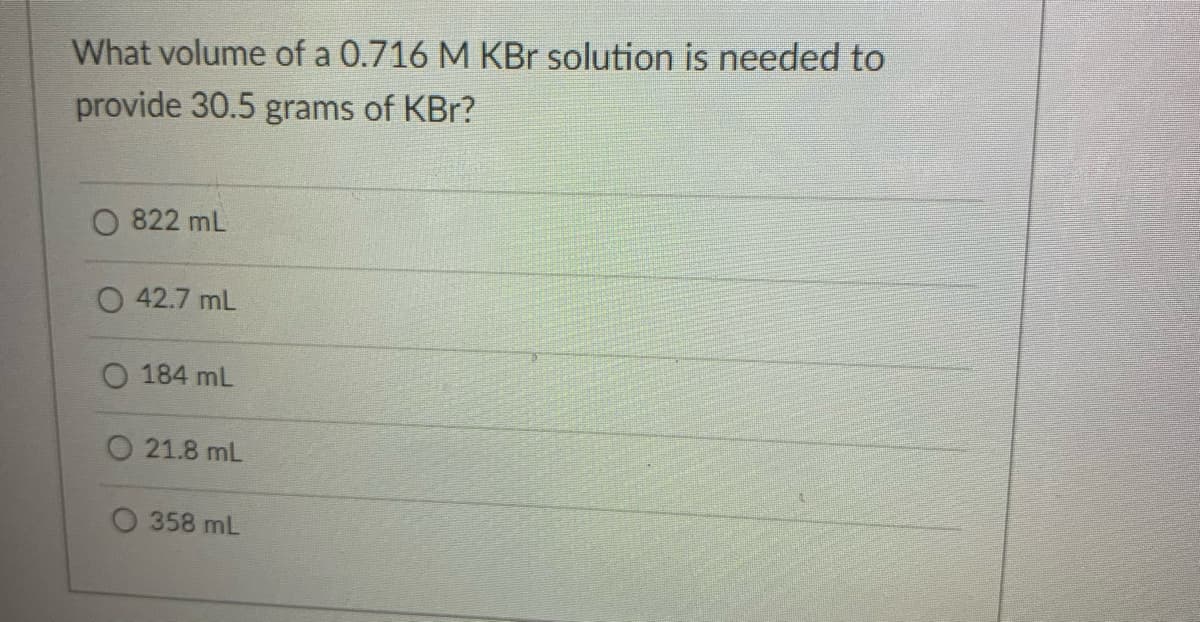
Transcribed Image Text:What volume of a 0.716 M KBr solution is needed to
provide 30.5 grams of KBr?
822 mL
O 42.7 mL
O 184 mL
O 21.8 mL
358 mL
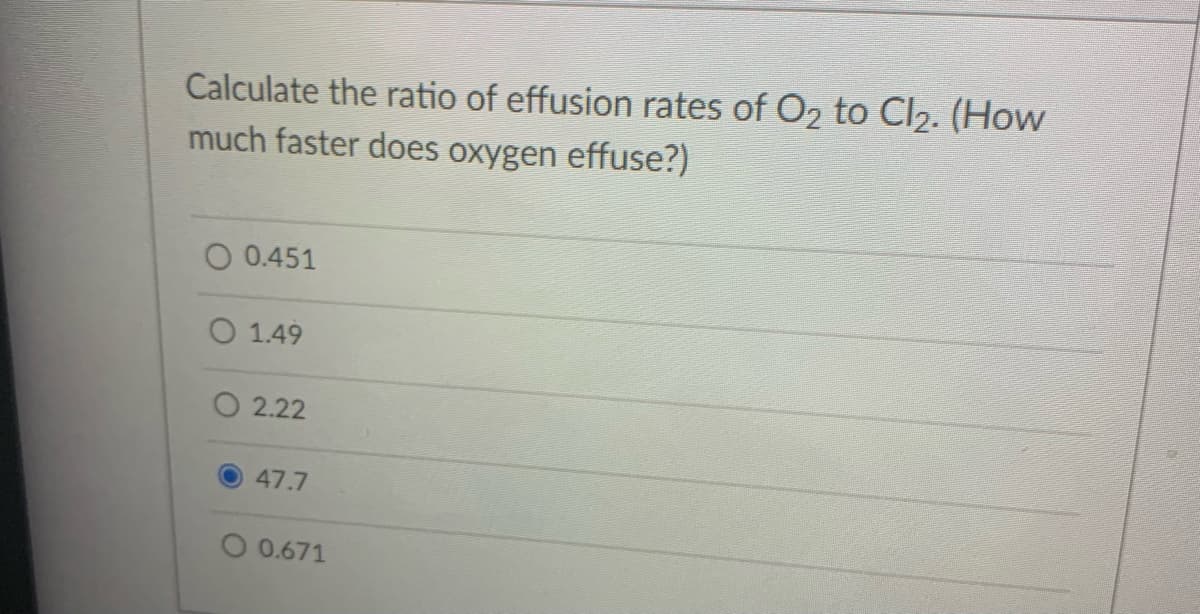
Transcribed Image Text:Calculate the ratio of effusion rates of O2 to Cl2. (How
much faster does oxygen effuse?)
0.451
O 1.49
O 2.22
47.7
O 0.671
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
Expert Answers to Latest Homework Questions
Q: Power point of John Nash
script for the presentation.
Remember to give proper credit to your…
Q: This aldol reaction product:
Thermodynamic enolate
OH
was made using a (circle one):
Kinetic enolate
Q: f(x1,x2)
4
2
0
-2
-4
−1
2
0
1
1
0
2
-1
X1
3-2
x2
Figure 2: Graph of the function f(x1, x2) = 2x1 =…
Q: Complete the following table by filling in missing amounts.
Note: Use 360 days a year.
$
Principal…
Q: AASB 116 requires disclosure of the following by asset class:
I
II
III
IV
The useful lives of assets…
Q: Using the data calculate the balance on current account for this economy.
The value of the current…
Q: 1
16
On January 1, 2024, Clor-Proell Enterprises bought 20% of the outstanding common stock of Chen…
Q: Required information
[The following information applies to the questions displayed below.]
High Time…
Q: 5. Circle all of the following species that exhibit geometric isomers. (3 pts total)
(a)…
Q: Using the forward price approach to finish the following blanks. The expected closing basis
was…
Q: Vaughn Manufacturing's budgeted manufacturing costs for 50000 squares of shingles are as follows:…
Q: Please answer in typing format
Q: During a titration, 0.5 M NaOH is added to 25.0 mL of 0.7 mol/L HF until the equivalence
point is…
Q: A firm has sales of $1,040, net income of $208, net fixed assets of $508, and current assets of…
Q: 13. Draw a line structure for the organic product of
the reaction shown below, which takes place in…
Q: Helppp please answer this as fast as possible
In google sheets how do I make my graph look like…
Q: 4. A solution is prepared by titrating a 100.0 mL sample of 0.10 M HF (Ka = 7.2 \times
10-4) with…
Q: Please answer in typing format
Q: The color for each “hexaaqua” complex is given below. Select the ligands thatcould change the color…
Q: Total load 87 psf
=
B1W16 x 57
G1W24 x 68
Compute Vmax and Mmax for Beam 1 and Girder 1 in the roof…
Q: What is the best prediction of the number of countries that participate in the Olympics in 1980?