Q: 2. The full structural formulae of three organic compounds, P, Q and R, are shown below. H H H H HH ...
A: Isomers are those which have same molecular formula but different arrangement of atoms and molecules...
Q: This drug is likely to bind to what protein? 2. Identify the classification of this drug 3. Name t...
A:
Q: What is the difference between the excitation and emission spectrum?
A: Difference between excitation and emission spectrum.
Q: (a) Take [Fe2-] = [Fe3+] = 102 mol/litre. Calculate logPO2 at pH = 1 for the following reactions: (i...
A: GIVEN: Fe2+ = Fe3+ = 10-2 mol/L pH= 1 R=8.314 J/mol K Temperature = 25oC = 298K To Calculate: log PO...
Q: alcohol
A:
Q: 2) Draw the structures for the following compounds. (a) trans-l-methyl-3-propylcyclobutane (b) 4,5-d...
A:
Q: When 60.0 mL of a 0.400 M solution of HN03 (ag) is combined with 60.0 mL of a 0.400 M solution of Na...
A: The volume of the final solution is = 60.0 mL + 60.0 mL = 120.0 mL The density of the solution is = ...
Q: Enter your answer in the provided box. Calculate the molarity of 8.25 mL of 0.0456 M KI diluted to 1...
A: The solution is as follows:
Q: What contributes to a group being either electron-donating or withdrawing? Select all that apply. ...
A: Electron donating groups are those which increases electron density on ring.
Q: A candle snuffer is a long handle with a small bell-shaped piece of metal on the end of it that is u...
A: Here we have to find the limiting reactant in the combustion reaction happening at the candle wick. ...
Q: OH H", heat HO H,0 H,0 Reduction [이 [이
A:
Q: EXERCISE II-8 Identify which indicator will be suitable for the following titrimetric analyses: a. 0...
A: The answer is as follows:
Q: What molality of NaCl is required to increase the boiling point of 500 g of water to 105°C? (K, is o...
A: Given: The boiling point of the solution = 105°C Mass of water = 500 g Standard data: Kb for wate...
Q: buffer solution is prepared by dissolving 4.7 g of nitrous acid, HNO2 , and 13.8 g of sodium nitrite...
A: The solution is as follows:
Q: Choose the best set of conditions for a Friedel-Crafts acylation of aromatic compounds. A) CH3C(O)CI...
A: Friedel-Crafts Reaction
Q: provicde three alkyl halide and discuss its structure
A: Alkyl halide are those in which carbon is attached to halogen atom.
Q: Kerosene has a specific weight of γ_k = 51.1 lb/ft³ and benzene has a specific weight of γ_b = 56.2 ...
A: Density of substance is calculated by dividing mass with volume.
Q: Consider a hypothetical case in which the charge on a proton is twice that of an electron. Using thi...
A: Answer: Potassium-39 means, its mass number is 39. Also neutron is always a neutral particle.
Q: 2. Twenty-five mL of 0.100M lactic acid (K = 1.4 x 104) is titrated with 0.097 M KOH. a) What is the...
A: Soln
Q: In each scenario, describe a step by step process in determining the unknown. Distilled water maybe ...
A: When a substance is prone to absorption of moisture on exposure to the environment, then it is terme...
Q: What is the initial mass of copper (in g) in the Cu(NO3)2 solution. Cu(NO3)2(aq)+ 2NaOH(aq)-> 2NaNO3...
A: Ans. 63.5 g of Cu is present in Cu(NO3)2 solution
Q: Analysis of a mixture consisting of NaOH + NażCO3 + inert matter gives the following data: Sample po...
A: Mass of original sample = 10.00 g Volume of actual solution made with original sample = 250.0 mL Vol...
Q: Enter your answer in the provided box. Calculate the molality of a solution containing 281 g of HCl ...
A: Molality: The mole of the solute dissolved in one kilogram of the solution is known as the molality ...
Q: Identify the false statement with regard to electromagnetic radiation and the electromagnetic spectr...
A:
Q: Please help me with both questions
A: The correct options are:
Q: Hydrogen and oxygen react to form water, together they are a .... - molecule - compound - neutro...
A: Hydrogen and oxygen are atoms that combine to form water
Q: Give a definition of what polymers are, and what is their application or use in everyday life
A: Answer of the question given below,
Q: 8. Give IUPAC names for the following compounds: (a) CI „Br (b) CH3 (c) NH2 CH2CH2CHCH3 Br (d) CI CH...
A: IUPAC nomenclature is used for naming organic compounds. Full form of IUPAC is international union o...
Q: What is t decimal praces. Answer should not include a unit label or other "lar mass of NH,CI? Round ...
A: Molecular mass: It can be defined as the sum of the atomic mass of each atom in the molecule. 1) fir...
Q: Answer the following question with respect to Fatty Acid Methyl Esters (FAMEs) by Gas Chromatography...
A: Fatty acids of food product is determined by derivatization Of fatty acids in its ester components.
Q: 0 Calculate the ionic strength for a solution containing 0.01SM NaCl and 0.0ZM CaSO Estimate the act...
A: A question based on ionic equilibrium that is to be accomplished.
Q: Question: 1, Based on Beer-Lambert law, A = ɛ. c. L, where A, absorbance; ɛ, extinction coefficient;...
A:
Q: If you blow carbon dioxide gas into a solution of calcium hydroxide, a milky-white precipitate of ca...
A: When we blow carbon dioxide gas into a solution of calcium hydroxide, a milky-white precipitate of c...
Q: Predict the reactants to synthesize the following. Draw their mechanism
A: Since you have asked multiple question, we will solve the first question for you. If you want any sp...
Q: Nitrogen monoxide, NO, reacts with hydrogen to give nitrous oxide, N20, and water. 2NO(g) + H2 (g) →...
A:
Q: Which of the following is not composed of a sugar? * O Plants O Beef Fats Water O DNA
A: 1. Answer is (c) - water a. plants contain sucrose,fructose,glucose naturally
Q: 1. Convert following retrosynthesis to synthesis (give all necessary steps, reagents and products af...
A: Since you have posted multiple questions, we will answer the first one for you. To get the remaining...
Q: Henderson-Hasselbach equation b) Calculate the pH of a buffer solution that contains 0.0416 M sodiu...
A:
Q: Choose the best answer given: Suppose you apply a flame to 1 L of water for a certain time and its t...
A: Thermodynamics is branch of the chemistry in which we deal with heat absorbed or gained by system.
Q: The diffusion coefficient of water molecules in hydrogen (the carrier gas) at 307 k and 1 atm is 1 c...
A: Diffusion is phenomenon that takes place where there is a concentration gradient. A concentration gr...
Q: conc. H2SO4 180 °C HO
A: The dehydration reaction of alcohols to generate an alkene. Dehydration means removal of water. De...
Q: How many molecules of C2H6 are there in 21.4 g of C2H6?
A: Given that, 21.4 g of ethane, C2H6. We have to calculate the number of molecules in it.
Q: A 1.98 g sample of nitrous oxide (an anesthetic sometimes called laughing gas) contains 2.71 x 1022 ...
A:
Q: 2. Write the structural formula of the major organic product formed in each of the following reactio...
A: a) The product formed from the given reaction is 2-methyl pentan-3-ol.
Q: What are the names of the following compounds? a. HNO2 (aq) Name: b. SO2 Name: с. Cu(Н, РОд)2 Name: ...
A:
Q: Directions: Explain your answer comprehensively. Limit your justification in 3-5 sentences. 1. All H...
A: According to Hess's Law of Constant Heat Summation The total enthalpy change for the reaction is the...
Q: 7. Proteins and carbohydrates consist primarily of carbon, hydrogen, and oxygen; these two molecules...
A: 1) Each atom has a nucleus made up of protons and neutrons. 2) In atoms, the number of protons...
Q: A chemist vaporizes a volatile liquid hydrocarbon (carbon, hydrogen and oxygen) compound to a gas an...
A:
Q: 4. Predict the pH of a solution that is made by mixing 25.00 mL of 0.100 M NH4OH (Kp = 1.80 x 105) w...
A: Given, 25 ml of 0.1 M NH4OH And 17.50 ml of 0.1 M HCl Kb = 1.80x10-5 pKb = - log(1.80x10-5) ...
Q: Calculate the molarity of 39.0 g of table sugar (C12H2201) in 100. mL of solution. M
A:
A CHEM student is investigating the equilibrium of iron thiocyanate:
Their reaction mixture is prepared by mixing together the following reagents:
- 2.50 mL of 0.002451 M Fe(NO3)3
- 6.0 mL of 0.1 M HNO3
- 1.50 mL of 0.0026721 M KSCN
From literature, you know that Kc is 134 at 20°C. What will the [Fe(SCN)2+] be at equilibrium, in M?
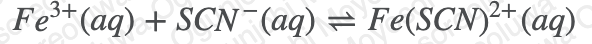
Step by step
Solved in 2 steps

- In the reaction AgNO3(aq) + KI(aq) → AgI(s) + KNO3(aq) which ions are the spectator ions?In the following reaction, what ions (if any) are spectator ions? AgNO3 (aq) + 2NaI (aq) -> AgI (s) + NaNO3 (aq)for the reaction 2Mg(s) + Cu(NO3)2 (aq) -> Cu(s) + 2 MgNO3 (aq) write a net ionic equation, which reactant was oxidized, which reactant was reduced, which reactant is the reducing agent, which reactant is the oxidizing agent?
- Cu(s) + NO3-(aq) → Cu2+(aq) + NO2(g) Referring to the equation above, which is the reducing agent?What is the balanced net ionic chemical equation between Cu^2+ (aq) and I^- (aq)?1-What is the missing product in the following chemical reaction ? 2-Which of the following is the correct balancing coefficients ? ___Ag NO3(aq) + ____CaCl2 (aq) → AgCl (s) +_________(aq)
- Silver is often extracted from ores such as K[Ag(CN)2] and then recovered by the reaction: (10pts) 2K [Ag(CN) 2] (aq) + Zn(s) 2Ag(s) + Zn(CN)2(aq) + 2KCN(aq) What mass of Zn(CN)2 is produced?What ions (if any) are spectator ions? AgNO3 (aq) + 2NaI (aq) AgI (s) + NaNO3 (aq)The mass of a solid piece of iron Fe(s) should be determined by allowing it to react with a solution of 0.500 L potassium dichromate K2Cr2O7 (aq) in a redox reaction in acidic solution, so that all iron is oxidized into iron (II) ions, Fe2+(aq). Concomitant chromium(III) ions, Cr3+(aq), after the reduction equation: Cr2O7 2−(aq) + 14H +(aq) + 6e − → 2Cr3+(aq) + 7H2O(l) a) Write the complete and balanced reaction equation for the redox reaction where the piece of iron is oxidized into iron (II) ions by the dithromations in the solution. b) A sample of the reaction solution after the oxidation of iron is completed shows that the molar concentration of Cr3+ ions is 0.0162 M. What is the calculated mass of the piece of iron Fe(s) in grams? c) What is the amount of electrons e − in moles transmitted in this specific reaction? Calculate the electrical work wel the oxidation of the piece of iron has created in kilojoules (kJ), using the enclosed electrode potentials E 0 and the…
- A chemist performs a gravimetric analysis. The chemist combines 1.00L of 2.00MAgNO3(aq) with 1.00L of 4.00MNaCl(aq) in an Erlenmeyer flask. Both the AgNO3(aq) solution and the NaCl(aq) solution are colorless. After the mixture has been stirred, a cloudy white substance is observed at the bottom of the flask. What is the expected mass of AgCl(s)AgCl(s), in grams, assuming that the yield is 100%100%? In the box, enter the mass to the nearest gram. gTo measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.3600M silver nitrate AgNO3 solution to a 24.00g sample of the fluid and collects the solid silver chloride AgCl product. When no more AgCl is produced, he filters, washes and weighs it, and finds that 1.28g has been produced. The balanced chemical equation for the reaction is: Cl−(aq) + AgNO3(aq) -> AgCl(s) + NO−3(aq) What kind of reaction is this? If you said this was a precipitation reaction, enter the chemical formula of the precipitate. If you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base. If you said this was a redox reaction, enter the chemical symbol of the element that is oxidized. Calculate the mass percent of Cl in the sample. Be sure your answer has the correct number of significant digits.___Na(s) + ___Cl2(g) -> ___NaCl(aq) What are the coefficients needed to balance the following equation?

