What would be the molar ratio of cyanic acid (HCNO)/sodium cyanate (NaCNO) buffer having a pH of 4.80 ? [K₂(HCNO) = 2.0 × 10-4 O 10.6 mol NaCNO to 1.0 mol HCNO O 11.6 mol NaCNO to 1.0 mol HCNO O 12.6 mol NaCNO to 1.0 mol HCNO O 13.6 mol NaCNO to 1.0 mol HCNO O None of the above
What would be the molar ratio of cyanic acid (HCNO)/sodium cyanate (NaCNO) buffer having a pH of 4.80 ? [K₂(HCNO) = 2.0 × 10-4 O 10.6 mol NaCNO to 1.0 mol HCNO O 11.6 mol NaCNO to 1.0 mol HCNO O 12.6 mol NaCNO to 1.0 mol HCNO O 13.6 mol NaCNO to 1.0 mol HCNO O None of the above
Chapter15: Acid-base Equilibria
Section: Chapter Questions
Problem 59E: Consider the titration of 100.0 mL of 0.200 M acetic acid (Ka = 1.8 105) by 0.100 M KOH. Calculate...
Related questions
Question
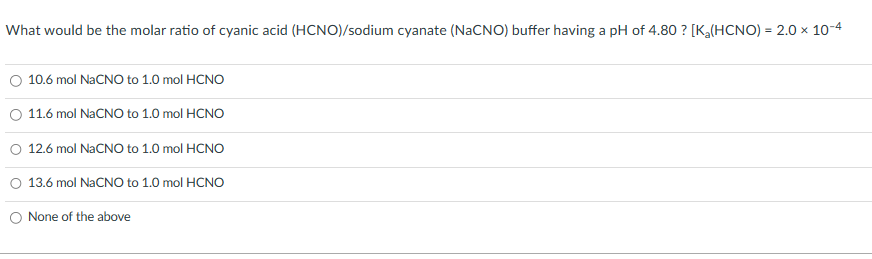
Transcribed Image Text:What would be the molar ratio of cyanic acid (HCNO)/sodium cyanate (NaCNO) buffer having a pH of 4.80 ? [K₂(HCNO) = 2.0 × 10-4
10.6 mol NaCNO to 1.0 mol HCNO
11.6 mol NaCNO to 1.0 mol HCNO
12.6 mol NaCNO to 1.0 mol HCNO
O 13.6 mol NaCNO to 1.0 mol HCNO
None of the above
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
