When 25.0 mL of a 8.39x10 M potassium phosphate solution is combined with 12.0 ml. of a 4.85X10M calcium chloride solution does a precipitate form? |(yes or no) For these conditions the Reaction Quotient, Q, is equal to
When 25.0 mL of a 8.39x10 M potassium phosphate solution is combined with 12.0 ml. of a 4.85X10M calcium chloride solution does a precipitate form? |(yes or no) For these conditions the Reaction Quotient, Q, is equal to
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter16: Solubility And Precipitation Equilibria
Section: Chapter Questions
Problem 21P
Related questions
Question
Solve both please ASAP
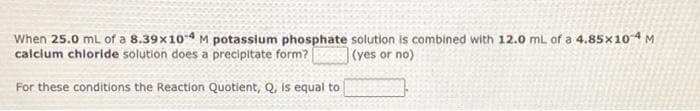
Transcribed Image Text:When 25.0 mL of a 8.39x104 M potasslum phosphate solution is combined with 12.0 mL of a 4.85x104 M
calclum chloride solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
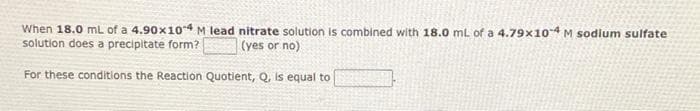
Transcribed Image Text:When 18.0 mL of a 4.90x1o4 M lead nitrate solution is combined with 18.0 mL of a 4.79x104 M sodlum sulfate
solution does a precipitate form?
(yes or no)
For these conditions the Reaction Quotient, Q, is equal to
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning