When baking soda reacts with acetic acid, what is the mole to mole ratio between baking soda and the gaseous product. See the chemical reaction below for reference. Make sure that it is balanced! NaHCO3(aq) + HC2H3O2(aq) → CO2(g) + H2O(I) + NaC2H3O2(aq) a) 1:0 b) 1:3 c) 1:2 d) 1:1
When baking soda reacts with acetic acid, what is the mole to mole ratio between baking soda and the gaseous product. See the chemical reaction below for reference. Make sure that it is balanced! NaHCO3(aq) + HC2H3O2(aq) → CO2(g) + H2O(I) + NaC2H3O2(aq) a) 1:0 b) 1:3 c) 1:2 d) 1:1
Chapter2: Decimal Fractions
Section: Chapter Questions
Problem 1RP
Related questions
Question
please explain your answer
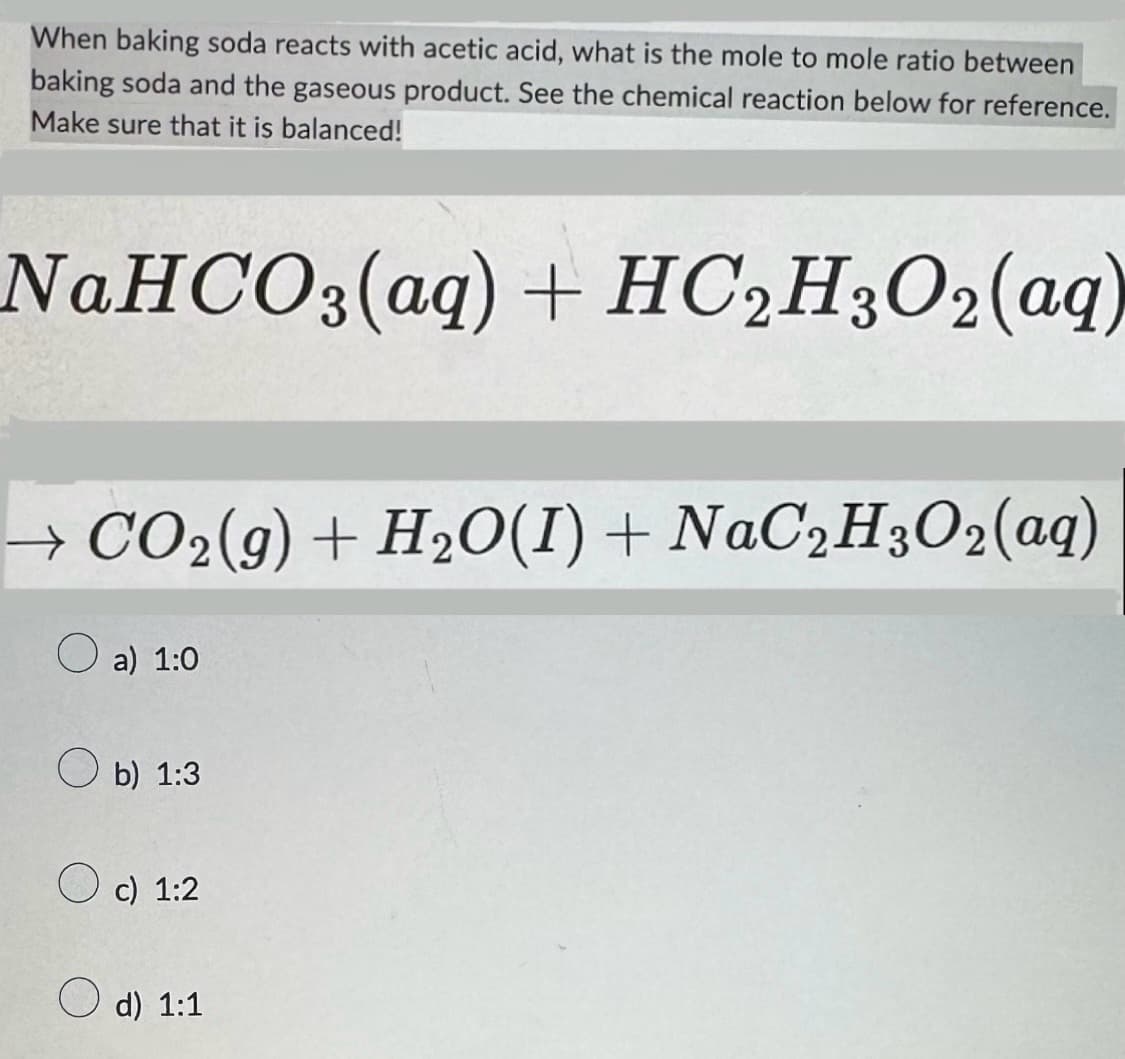
Transcribed Image Text:When baking soda reacts with acetic acid, what is the mole to mole ratio between
baking soda and the gaseous product. See the chemical reaction below for reference.
Make sure that it is balanced!
NaHCO3(aq) + HC2H3O2(aq)
→ CO2(g) + H2O(I) + NaC2H3O2(aq)
a) 1:0
b) 1:3
c) 1:2
d) 1:1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you





