Based on the Henderson-Hasselbalch equation (shown below), calculate the pH when half of a solution of acetic acid is dissociated to acetate (the pKa of acetic acid is 4.76). A. 1.00 B. 3.76 C. 4.76 D. 5.76
Based on the Henderson-Hasselbalch equation (shown below), calculate the pH when half of a solution of acetic acid is dissociated to acetate (the pKa of acetic acid is 4.76). A. 1.00 B. 3.76 C. 4.76 D. 5.76
Basic Clinical Laboratory Techniques 6E
6th Edition
ISBN:9781133893943
Author:ESTRIDGE
Publisher:ESTRIDGE
Chapter6: Basic Clinical Chemistry
Section6.1: Introduction To Clinical Chemistry
Problem 7RQ
Related questions
Question
Based on the Henderson-Hasselbalch equation (shown below), calculate the pH when half of a solution of acetic acid is dissociated to acetate (the pKa of acetic acid is 4.76).
A. 1.00
B. 3.76
C. 4.76
D. 5.76
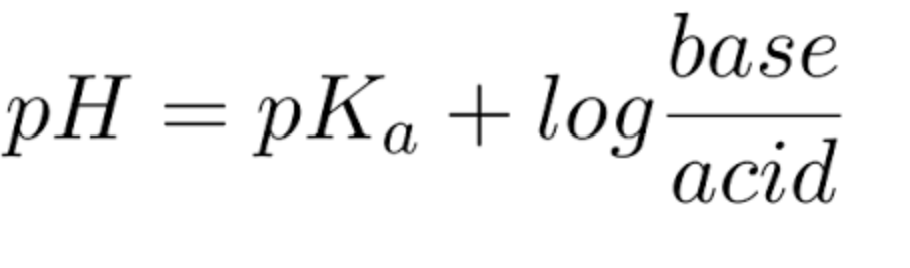
Transcribed Image Text:pH = pKa + log
base
acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Principles Of Radiographic Imaging: An Art And A …
Health & Nutrition
ISBN:
9781337711067
Author:
Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:
Cengage Learning



Principles Of Radiographic Imaging: An Art And A …
Health & Nutrition
ISBN:
9781337711067
Author:
Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:
Cengage Learning
