When comparing the structures of aspirin and acetaminophen, why can acetaminophen exist in a liquid state (i.e. childrens drinkable tylenol) and aspirin cannot? Does it have to do with the carboxylic acid? The question says that liquid aspirin cannot be made. Why would that be?
When comparing the structures of aspirin and acetaminophen, why can acetaminophen exist in a liquid state (i.e. childrens drinkable tylenol) and aspirin cannot? Does it have to do with the carboxylic acid? The question says that liquid aspirin cannot be made. Why would that be?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter1: The Nature Of Chemistry
Section: Chapter Questions
Problem 114QRT
Related questions
Question
When comparing the structures of aspirin and acetaminophen, why can acetaminophen exist in a liquid state (i.e. childrens drinkable tylenol) and aspirin cannot? Does it have to do with the carboxylic acid? The question says that liquid aspirin cannot be made. Why would that be?
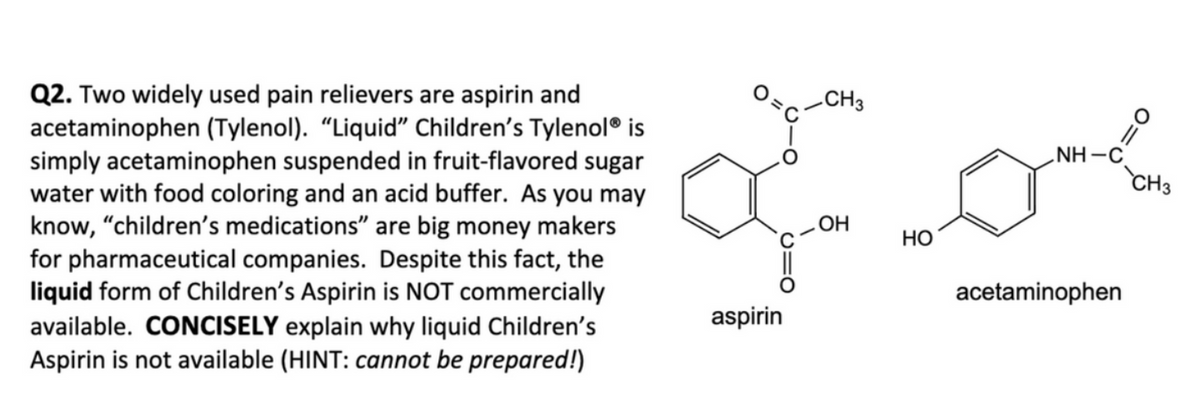
Transcribed Image Text:Q2. Two widely used pain relievers are aspirin and
acetaminophen (Tylenol). "Liquid" Children's Tylenol® is
simply acetaminophen suspended in fruit-flavored sugar
water with food coloring and an acid buffer. As you may
know, "children's medications" are big money makers
for pharmaceutical companies. Despite this fact, the
liquid form of Children's Aspirin is NOT commercially
available. CONCISELY explain why liquid Children's
Aspirin is not available (HINT: cannot be prepared!)
میں مری
aspirin
CH3
OH
HO
NH-C
acetaminophen
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning